Artificial tissue from the test tube
Human tissue is often needed to repair injuries or treat certain diseases. Complications arising through tissue engineering are rare, because replacement tissue is grown from the patient’s own cells. The Fraunhofer IGB is now certified to manufacture tissue for cartilage transplants.
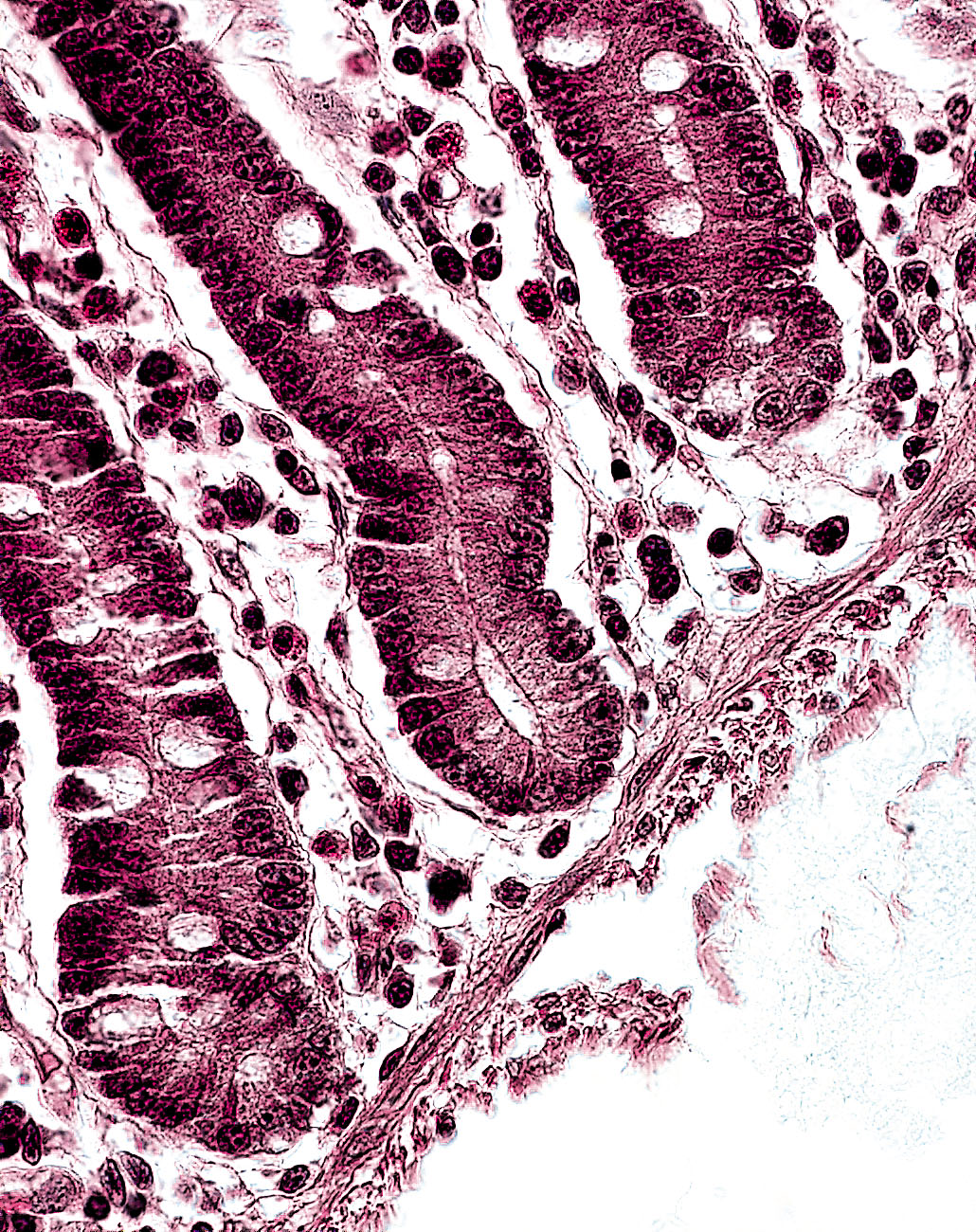
The human body is held together by collagen (from the Greek kolla = glue). This group of structural proteins makes up 20-30 percent of the protein content of mammals - and can be found in skin, cartilage, tendons, ligaments, blood vessels, teeth and bones. The range of associa-ted diseases is correspondingly wide. One example is osteoarthrosis, a degeneration of cartilage, the protecting tissue layer of the joint. To treat such pathologies or injuries, physicians need artificial tissue (or prostheses). As in any other transplant, the tissue is taken from another part of the body, from another patient, or from an animal donor. Particularly in the latter two cases, there is a high risk that the transplanted tissue will set off an immune reaction, leading to inflammation or rejection. By contrast, such complications can be avoided by an autologous, patient-specific transplant. This is achieved by using the relatively new biomedical technique of tissue engineering, in which the patient’s own cells are cultivated in the laboratory and used to manufacture the necessary tissue or cellular therapeutic.
The Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB in Stuttgart has now been certified for this type of work: One month ago, it was granted manufacturing approval under the German Medicines Act by the local drug surveillance board in Tübingen. “At the moment, this approval is limited to the manufacture of chondrocytes, or cartilage cells,“ says Dr. Hans-Georg Eckert, who heads the department for cell systems.“ This makes us one of a small circle of non-commercial laboratories in Germany allowed to manufacture such products for cell therapy.“ The experience gained during the certification process and the establishment of the required laboratory infrastructure will now be used for collaborative projects in this domain. The IGB scientists intend to expand their activity to include other types of cell and tissue, such as skin, bone, blood and nerve cells. With this range of services, the researchers hope in particular to serve specialist clinics, research institutes and small enterprises, which often are not equipped to meet the Good Manufacturing Practice (GMP) standards prescribed for clinical and pharmaceutical products.
“Once the tissue has been grown, it has to be tested to ensure that it is capable of performing the desired quality,“ remarks Dr. Ulrike Vettel, mentioning just one of the many laboratory tasks involved.“ In the case of cartilage we verify whether the cells produce the required type II collagen. If they don’t, the tissue is not released for clinical use.“
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB