Besides the antiviral assay (AVA) we perform a variety of assays to determine the titer of cytopathogenic viruses in various samples. Quality System Assay capabilities to suit the different development and regulatory needs are available, from R&D level to certified GLP standards.
Plaque Assay and Tissue Culture Infectious Dose50 (TCID50) Assay
The Plaque Assay
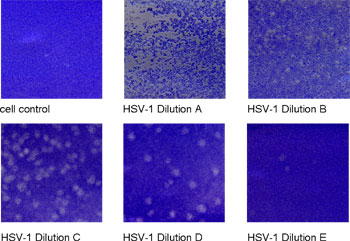
Plaque assay of Herpes simplex virus-1 (HSV-1) infected Vero B cells (staining with Coomassie).
Cytopathogenic viruses can be quantified by the number of plaques or pocks they cause on susceptible cell monolayers. Using this assay, we can screen drug compounds for plaque inhibition.
The Tissue Culture Infectious Dose50 (TCID50) Assay
Viruses which have cytopathic effect (CPE) can be quantitated using the TCID50 Assay. Endpoint techniques are used for viruses which do not grow in culture, when 'Lethal Dose50' (LD50) or 'Infectious Dose50' (ID50) values must be calculated.
They are also used in the case of viruses which are not cytopathic or do not produce plaques. We use several statistical methods for analyzing the data generated, e.g. Spearman-Karber analysis.
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB