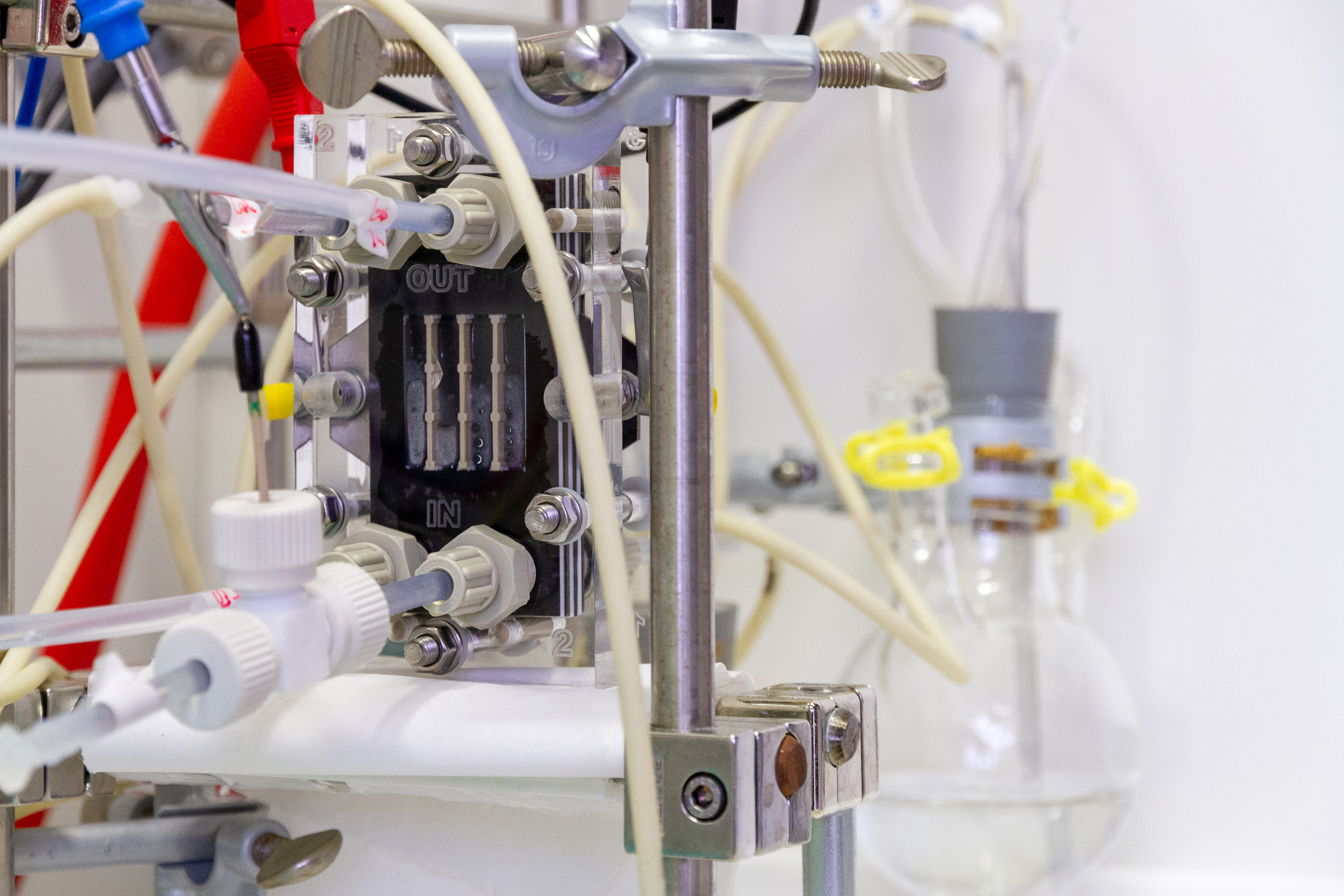
Catalysts for synthesis of ethylene through reduction of CO2, and hydrogen peroxide through oxidation of water
As part of the EU-funded CO2EXIDE project, the task of Fraunhofer IGB is to develop active and selective catalysts for simultaneously synthesising ethylene (C2H4) through cathodic reduction of CO2, and hydrogen peroxide through anodic oxidation of water. Both products are used in a subsequent reaction step to generate ethylene oxide (C2H4O). Thus, the CO2EXIDE approach enables the efficient production of three important platform chemicals, namely ethylene, hydrogen peroxide, and ethylene oxide, from the educts CO2, water and electric energy, hence from entirely renewable resources.
Literature
[1] Perry, S.C.; Pangotra, D.; Vieira, L. et al. (2019) Electrochemical synthesis of hydrogen peroxide from water and oxygen. Nat Rev Chem 3: 442–458. doi:10.1038/s41570-019-0110-6
Further publications within the project CO2Exide
Pangotra, D., Csepei, L.I., Roth, A. Ponce de León, C., Sieber, V., Vieira, L., (2022) Anodic production of hydrogen peroxide using commercial carbon materials, Applied Catalysis B: Environmental Vol: 303. https://doi.org/10.1016/j.apcatb.2021.120848
Rodin, V.; Lindorfer, J.; Böhm, H.; Vieira, L. (2020) Assessing the potential of carbon dioxide valorisation in Europe with focus on biogenic CO2. Journal of CO2 Utilization 41. https://doi.org/10.1016/j.jcou.2020.101219

 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB