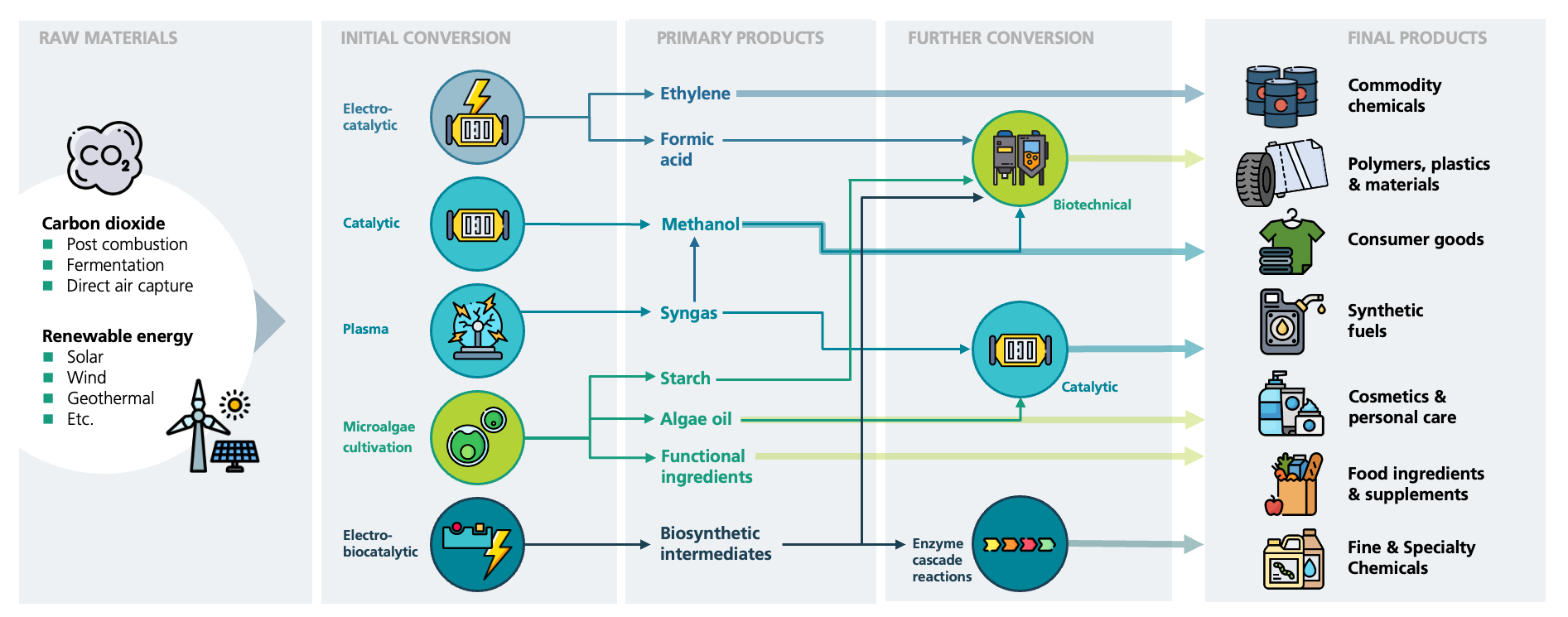
In order to stop further global warming, greenhouse gas emissions must be urgently avoided through savings in resource consumption and increased efficiency, but also by replacing fossil raw materials with renewable alternatives. One possibility is to use the greenhouse gas CO2 itself as a raw material for carbon-based products. At Fraunhofer IGB, we are pursuing various technological approaches to activate the inert CO2 molecule and subsequently use it to synthesize a variety of chemical products – from bulk to fine chemicals, from fuels to plastics.
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB