Breathing through mouth masks, substances from the mask material can enter the lungs via the inhaled air. To investigate the inhalation toxicological effects of such airborne substances, we adapted a reporter cell line used for identification of allergy- and asthma-inducing effects to special culture conditions and established an in-vitro test system using a culture and exposure system developed by Fraunhofer ITEM for the evaluation of airborne substances.
TOPAS-COVID19 – Investigation of the inhalation toxicological effects of face masks
In-vitro test system for standardized evaluation of respiratory toxicological effects caused by airborne substances
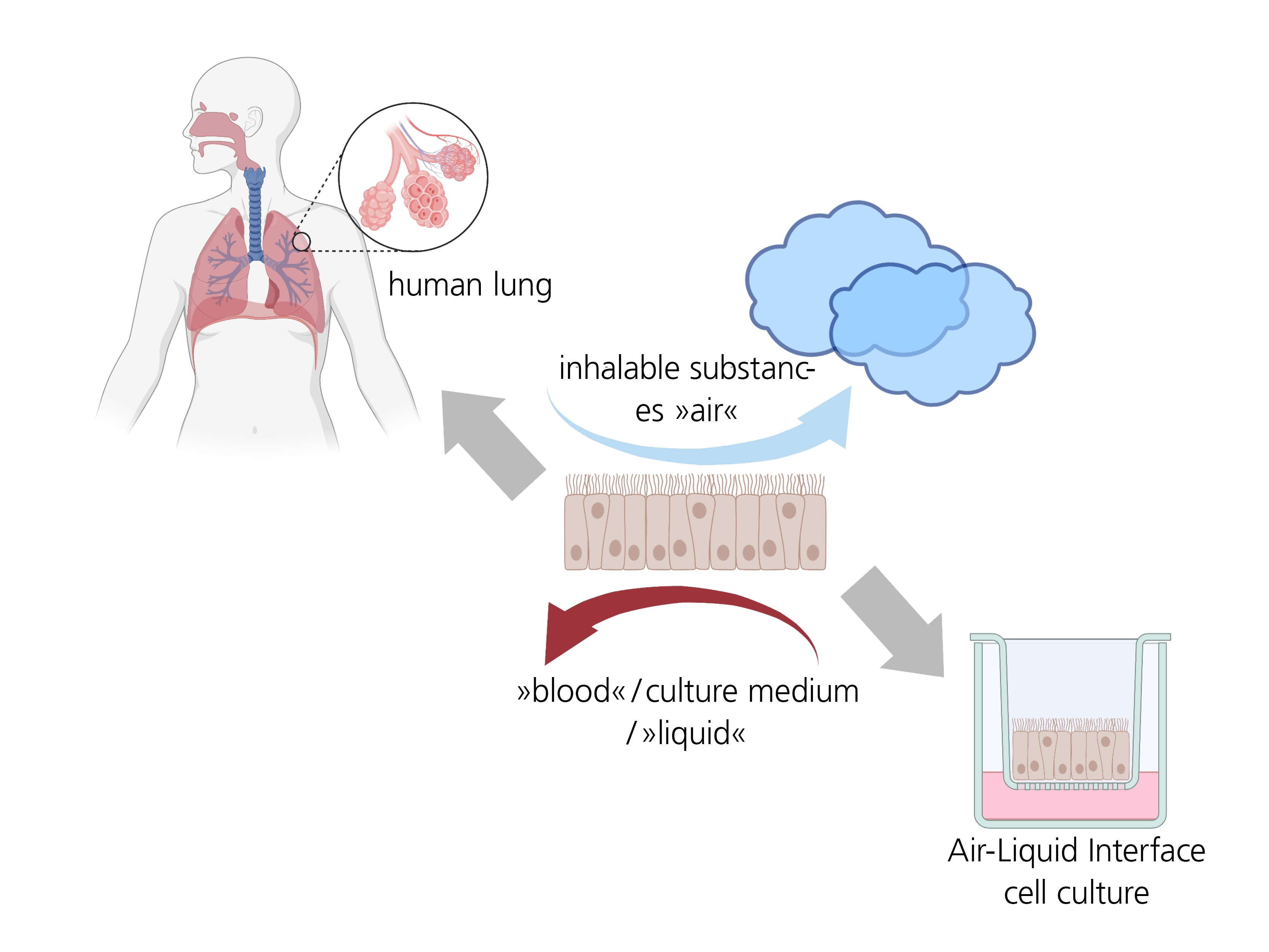
The requirements for medical face masks, such as filtration efficiency, breathability and biocompatibility, and the corresponding test methods are defined by legal test guidelines. The official standard for evaluating medical devices (DIN EN 10993) addresses biological effects, such as toxicity, irritation and allergy, resulting only from the medical device/material coming into contact with the skin or bodily fluids. Breathing through masks can trigger adverse respiratory toxicological effects caused by airborne substances that are released from the masks and then enter the lungs – even at very low concentration levels in the inhaled air. This can lead to long-term irritation or inflammation of the airways and even to allergies or asthma. A standard for evaluating airborne exposure in terms of respiratory toxicology does not exist.
Culture and exposure system for investigating airborne substances
Based on a culture and exposure system for airborne test substances patented by Fraunhofer ITEM, a unique in-vitro test battery to investigate the biological respiratory effects of face masks has been developed. The P.R.I.T.® ExpoCube® facilitates the exposure of cells at the air-liquid interface (air-liquid interface (ALI) cultures). This enables relevant endpoints of respiratory toxicological mechanisms to be investigated.
Reporter cell lines for identification of allergy- and asthma-inducing effects
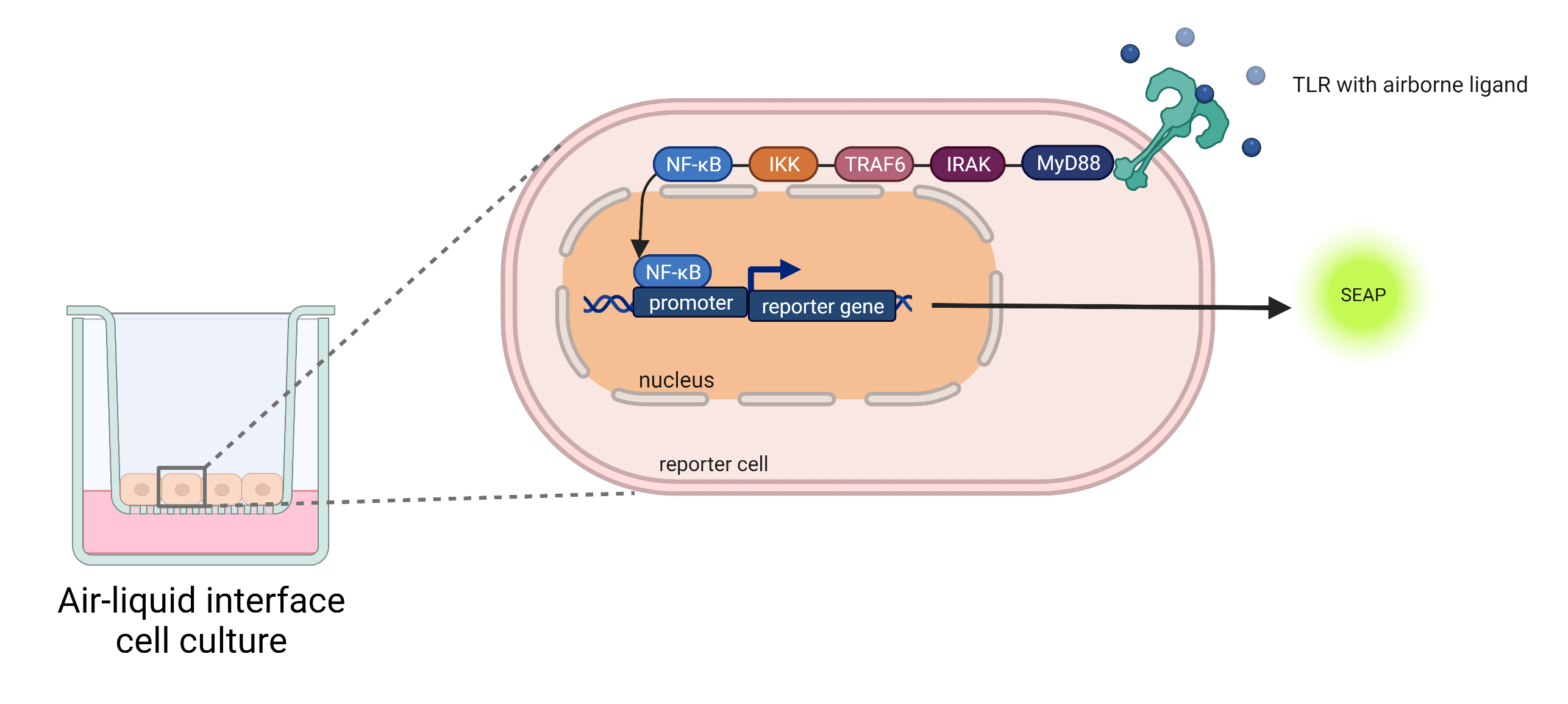
In addition to using commercial cell lines to evaluate toxic and/or irritative effects, our researchers in the “Cell and Tissue Technologies” innovation field have developed indicator cell lines, which can be used to study effects that result in the development of allergies and asthma. The basis for this are indicator cell lines (patented by Fraunhofer IGB – EP 2 041 172 B1) using human receptors from the innate immune system that are responsible for activating the immune system (toll-like receptors, TLR). TLR-activation of the reporter cells leads to reporter gene expression via intracellular signaling pathways. Receptor activation can be directly quantified via the reporter protein, in this case a secreted alkaline phosphatase (SEAP), by means of a color change reaction following the addition of a suitable substrate.
To be able to use the cells cultured under submerged conditions to investigate airborne substances for the first time, they were successfully adapted to the conditions of ALI cultivation. To complete the in-vitro test system, suitable vaporizable substances were also identified as positive controls as part of a screening procedure.
Demonstration of the functionality of the test system using a model contaminant
The functionality of the test system was successfully demonstrated through the use of formaldehyde as a model contaminant, supporting the potential application of the test system in the development of wearer-optimized protective masks suitable for daily use as part of the research project “TOPAS-COVID19.” On the basis of available limit values, a realistic test situation was successfully recreated not only conceptually but also in the quantitatively relevant concentration range of potential filter contaminants.
Project information
Project title
TOPAS-COVID19 – Everyday protective masks optimized for ergonomic use; Subproject: Development of an experimental approach for the investigation of inhalation-toxicological effects of protective masks suitable for everyday use
Project duration
December 2020 – November 2022
Project partners
- bagjack handmade, Berlin
- ORANGE Engineering Ost GmbH & Co.KG, Lichtentanne
- Spengler & Fürst GmbH & Co. KG, Crimmitschau
- Strick Zella GmbH & Co. KG, Zella
- Sächsisches Textilforschungsinstitut e.V., Chemnitz (Coordination)
- Fraunhofer IGB, Stuttgart
- Fraunhofer ITEM, Hannover
Funding
We would like to thank the German Federal Ministry of Education and Research (BMBF) for funding the project "TOPAS-COVID19", promotional reference 03COV13B.

 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB