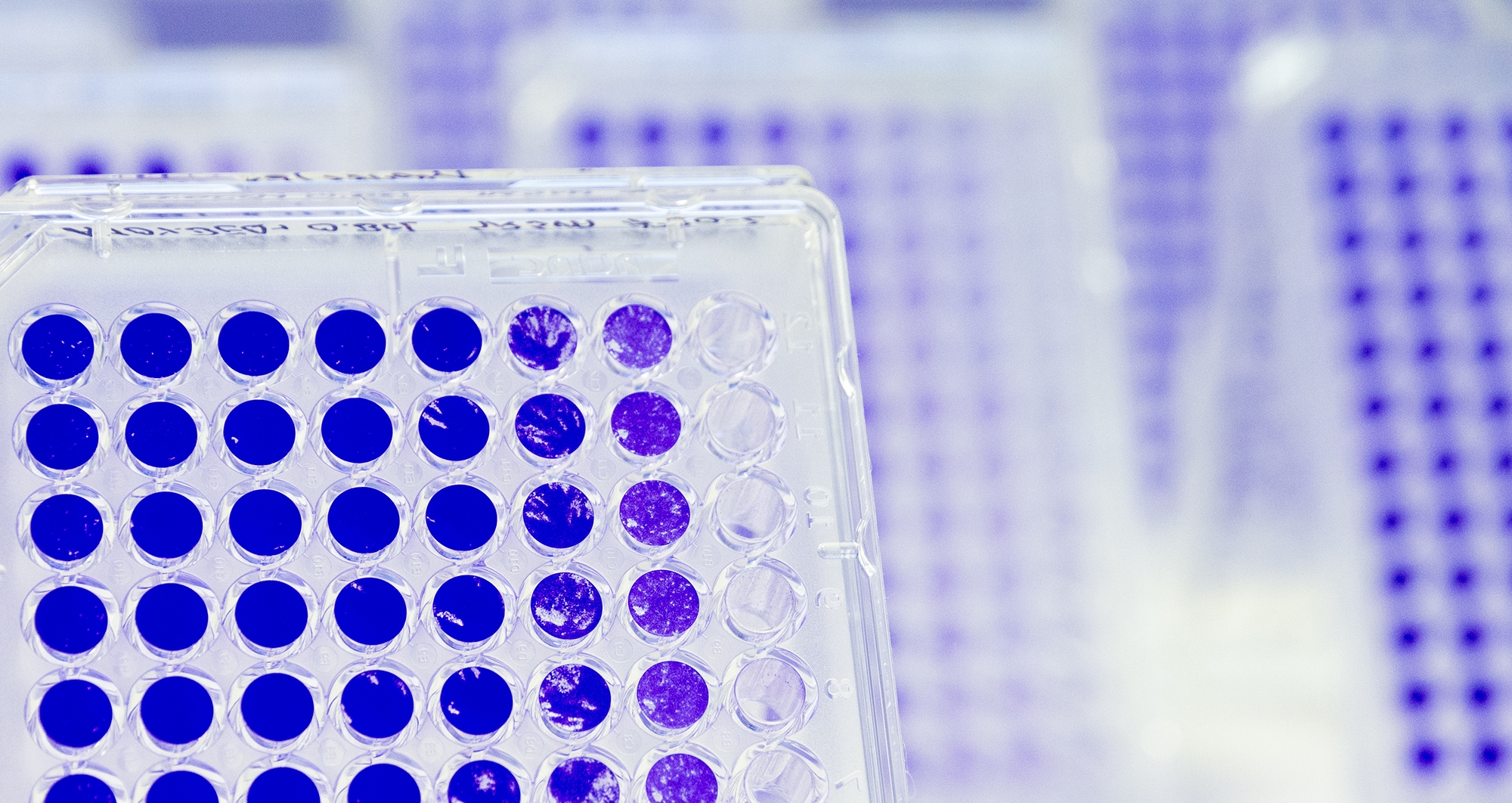
Viruses are tiny pathogens that threaten humans and animals in great diversity and lead to diseases. Understanding their infection mechanisms, diagnosing them and developing approaches for targeted prevention (vaccines) and therapy (antiviral molecules) are central concerns of our research group.
With our virological, cell biological and molecular biological experience and infrastructure we offer the cultivation, production and detection of selected human pathogenic viruses. Direct and indirect detection methods are available for characterization and further investigation. On this basis, we develop virus-based technologies to make innovative therapies available.
Virus culture and virus detection
Services

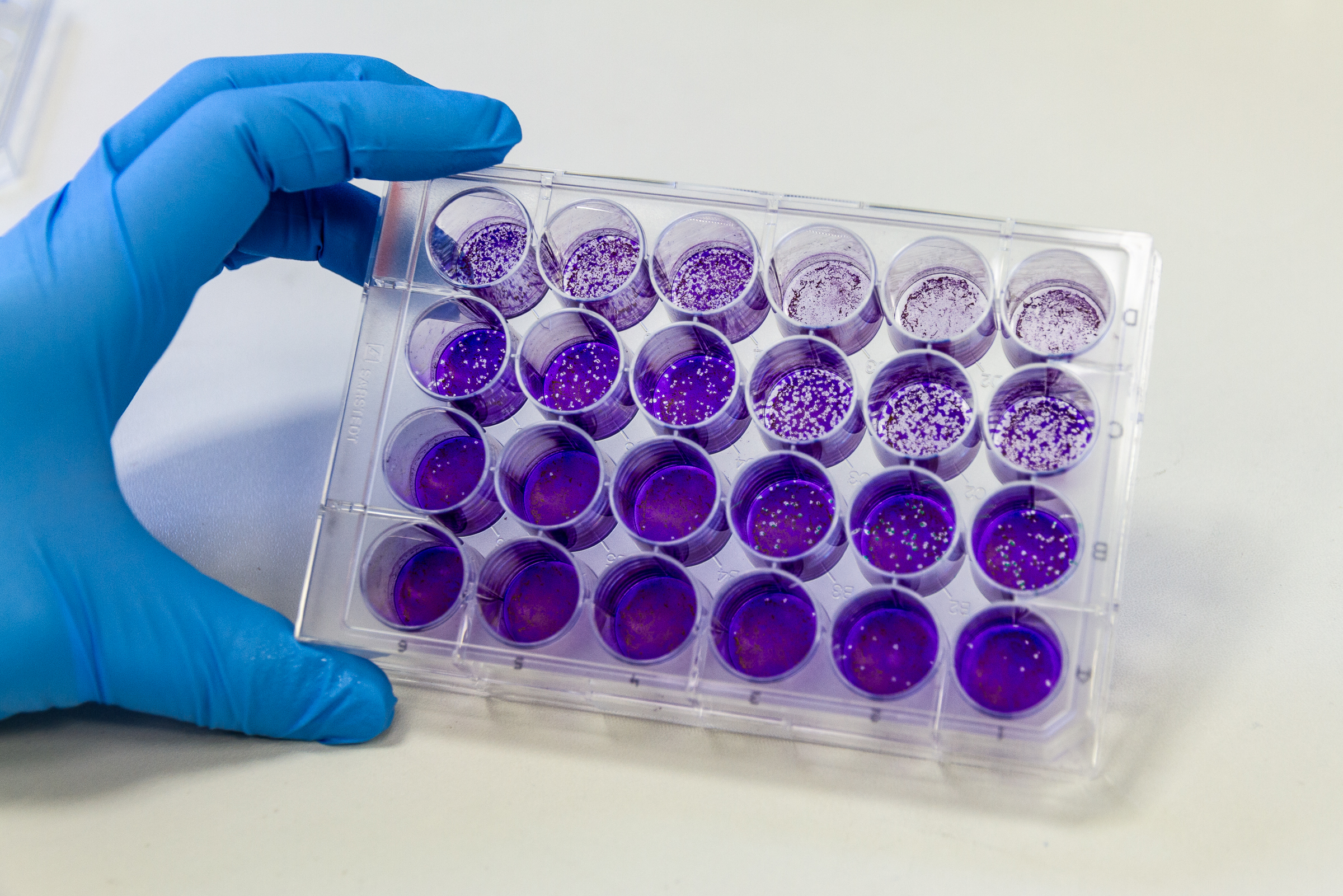

- Virus culture (quantification, purification, characterization of viruses)
- Testing of antiviral molecules and characterization of their mode of action
- Analysis of virus-virus and virus-host protein interactions
- 2D infection systems
- Virus diagnostics using bioanalyzer, PCR, RT-qPCR, microarray and ELISA
- Differentiation of bacterial, viral and fungal diseases
- Virus engineering (herpes viruses)
Viruses: Herpes viruses (HSV-1, HSV-2, VZV, EBV), polioviruses, influenza viruses can be produced, concentrated and purified in the L2/S2 range.
A number of virological assays are available for the characterization of the viruses, such as viral plaque assay, TCID50 assay, analysis of growth kinetics and ELISA.
For the detection of pathogen genomes, molecular biological assays such as bioanalyzer, PCR and RT-qPCR are available.
For the analysis of protein interactions (also in high throughput) the yeast 2-hybrid system, the Lumier and the BRET assay are used. Indirect immunofluorescence analysis using confocal microscopy is one of the standard methods.
Selected publications
Lieber, D.; Bailer S. M. (2013)
Determination of HSV-1 infectivity by plaque assay and a luciferase reporter cell line
Methods Mol Biol. 1064: 171-181. doi: 10.1007/978-1-62703-601-6_12.
Striebinger, H.; Koegl, M.; Bailer, S. M. (2013)
A high-throughput yeast two-hybrid protocol to determine virus-host protein interactions
Methods Mol Biol. 1064: 1-15. doi: 10.1007/978-1-62703-601-6_1.
Lenac Roviš, T.; Bailer, S. M.; Pothineni, V. R.; Ouwendijk, W. J.; Šimić, H., Babić, M.; Miklić, K.; Malić, S.; Verweij, M. C.; Baiker, A.; Gonzalez, O.; von Brunn, A.; Zimmer, R.; Früh, K.; Verjans, G. M., Jonjić, S; Haas, J. (2013)
Comprehensive analysis of varicella-zoster virus proteins using a new monoclonal antibody collection
J Virol. 87(12): 6943-6954. doi: 10.1128/JVI.00407-13.
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB