Bacteriophages are a widely used alternative to antibiotics in Eastern Europe. At the IGB we develop platforms to engineer and produce them. The body's immune system plays a central role in fighting infections. Research at Fraunhofer IGB therefore also focuses on immunomodulating agents and the cellular mechanisms that are set in motion during an infection.
Infections – Drug development
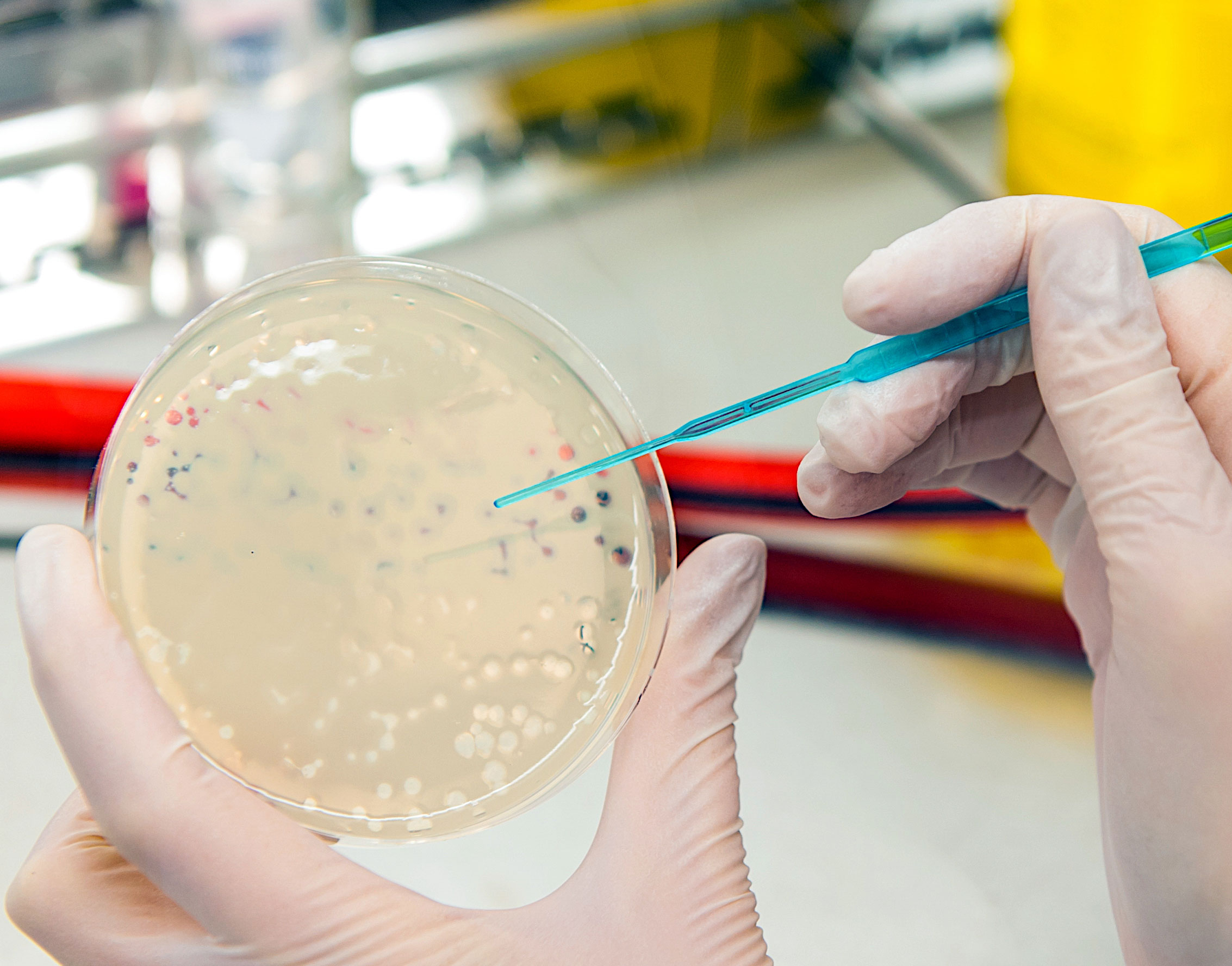
Bacteriophages – New weapon against multi-resistant bacteria
Bacteriophages are viruses that can specifically infect and kill bacteria. They have been used for a long time, particularly in Georgia, to treat infectious diseases, but have been replaced by antibiotics in Western Europe. Recently, great hopes have been placed in bacteriophages for the specific and effective treatment of bacterial infectious diseases.
The treatment of multi-resistant bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) or Extended Spectrum β-lactamase (ESBL) producing pathogens is of particular importance today. In this field, the IGB, together with Fraunhofer ITEM and IPA, is working to engineer bacteriophages and establish processes for their personalized production.
Experience in dealing with bacteriophages has already been gained in connection with the fight against caries, the number one chronic disease. The Kari-EX project has developed a completely new, cost-effective and safe method for the causal control of caries by integrating phages into chewing gum. Kari-EX thus paves the way for the use of phages in the fight against bacterially caused infectious diseases, especially by multi-resistant germs, where currently antibiotics are dramatically reaching their limits.
Identification of new agonists and antagonists of immune receptors for the therapy of inflammations

The use of immune receptor agonists and antagonists is a new therapeutic approach to treat pathologic inflammatory processes that play a role in infectious diseases (even sepsis), allergies, and autoimmune diseases. They are even involved in disposing tumor cells.
Receptor-agonists stimulate the innate immune system and are frequently used as drug adjuvants, while antagonists inhibit inflammation. Fraunhofer IGB has a number of mammalian whole-cell-biosensor-assays available that can measure the activity of all known human TLR-receptors and some C-lectines. This is done using spectroscopic methods in high-throughput screening (HTS). These methods successfully identify functional molecules that are potential drug candidates for the prevention and treatment of immunologic diseases.
After filing for a patent, first TLR9 antagonists are already being identified in cooperation with partners. By using whole cells or even tissue samples, simple receptor-ligand-interactions can be analyzed and very complex interactions within the cell or tissue aggregate can be recorded. In the meantime, development has progressed to the point that a patent was granted (Patent DE102011121556B4).
Immune competent 3D skin models
In vitro models allow the analysis of molecular processes in host-pathogen interactions. Especially epithelial models are suited for infection research to investigate the interaction between pathogens and host surfaces. This is because more severe courses of infection usually originate from microorganisms that naturally colonize the epithelial surfaces of the body such as skin, mucosa, and the gastrointestinal tract.
In our hospitals, infections with commensal facultative pathogenic microorganisms are one of the infectious diseases most difficult to control. Especially in nursing homes as well as in bedridden and intensive care patients, infections that are usually controllable by the body e.g. Staphylococcus aureus (MRSA) or Candida albicans lead to severe illness, often resulting in death.
To test infection mechanisms of these facultative pathogenic microorganisms, the IGB has developed in vitro infection models that can simulate infections in a human tissue model. The innate immune system decides especially at the human epithelial barrier whether a pathogen can invade and subsequently infect the body. The innate immune response against pathogens are strongly dependent on the communication of different cell types in a three-dimensional tissue structure.
This complex initial situation was the reason for the development of 3D infection models of the skin, which, in addition to the epithelial cells (keratinocytes), contain structural components such as dermal fibroblasts and collagen as well as immunologically relevant components such as different immune cell types. Such models have already been used to investigate mechanisms of host-pathogen-interactions. So far, especially infection processes in yeast (Candida spp.) as well as viruses (Herpes simplex-virus, HSV-1), and the immune response to these pathogens have been analyzed.
Using genome-wide analysis methods such as next-generation-sequence analyses, these infection models loaded with immune cells can be comprehensively analyzed. It was shown that no individual cell type alone effectively fights a pathogen, in this case C. albicans. Cytokine-mediated communication between the different cell types is necessary to launch an effective antimicrobial response by the dermal fibroblasts in the model. One of the key molecules is the immune receptor TLR2 that is necessary to recognize pathogens. It induces a signal cascade, which ultimately stops the yeast invasion.
These results underline the role of immune receptors as important sensors and regulators of the immune system. They help the body decide when and how to activate its immune mechanisms. Therefore, these models are optimally suited to identify and validate immunomodulatory substances that fight infections as well as immunologic diseases. For example, immune-activating substances can quickly eradicate an infection and immune suppressing components can reduce an excessive inflammatory reaction.
Using this strategy, the body’s arsenal against pathogens can be more strongly incorporated in therapeutic approaches. This would lead to quicker recovery from and better protection against infections.
These promising approaches to construct partially immune competent in vitro models will be expanded to better understand the molecular mechanisms of the body’s immune system on the epithelial level. Based on this, new drugs for treating infectious diseases will be developed. This is especially relevant for immune suppressed patients, whose immune system is weakened.

"Reporter skin" – Rapid optical detection of immune reactions during drug development
The interaction and communication between different epithelial cells and immune cells is essential to activate the targeted immune response against pathogens. However, misdirected communication leads to immune mediated diseases. Therefore, screening and validation systems that will quickly and efficiently identify and validate substances for these indications must be based on three-dimensional tissue equivalents.
To fulfill these conditions as closely as possible and based on the two previously mentioned articles, the IGB has developed a system that can optically capture an immune reaction in a three-dimensional tissue equivalent. For this purpose, systems that report receptor activation of the innate immune system (PRRs) were introduced into different cell types of the aforementioned 3D-skin model. Both transiently expressing primary cells and stably transfected immortalized cells can be implemented.
This 3D-reporter-skin model allows the optic/spectroscopic quantification of the activation and inhibition of central innate immune system signaling pathways in three-dimensional tissue aggregates (Patent DE102011121556B4).
Thus, the course of the infection as well as the effect of drugs on the course can be monitored “online“ in one or several tissue models. Complex infection processes can also be described at the epithelial barrier, and used for further drug development. This is achieved using different reporter and immune cells that are integrated into the system.
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB