Challenge: (pre-)clinical research without animal testing
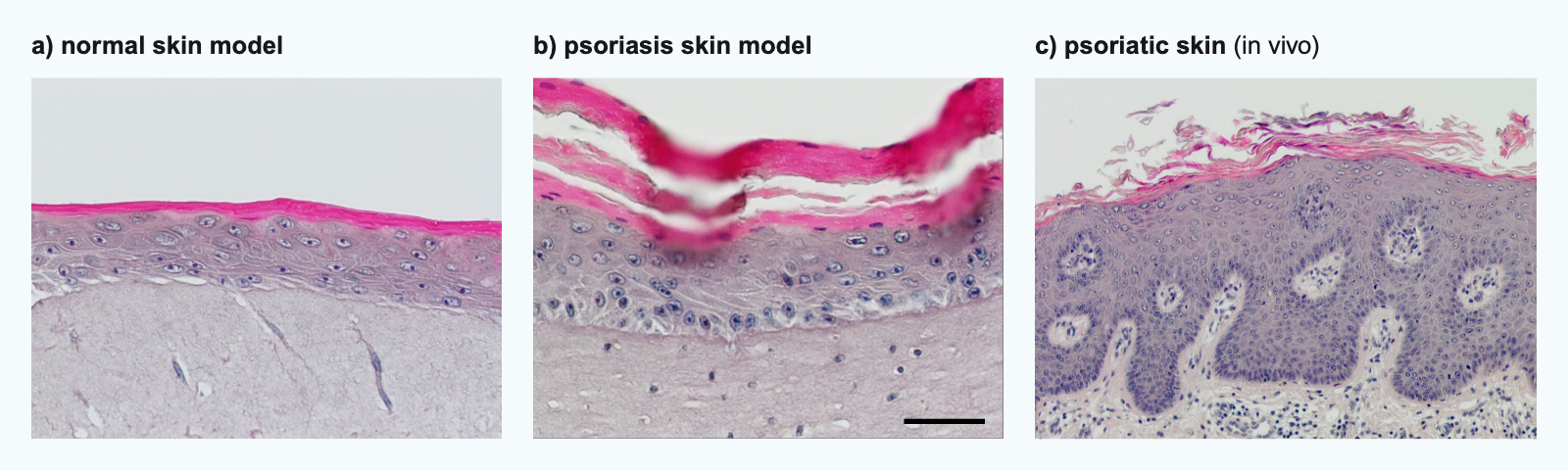
High failure rates in drug development are driving a paradigm shift in basic and preclinical research (Adhikary, P.P., Ul Ain, Q., Hocke, A.C. et al. COVID-19 highlights the model dilemma in biomedical research. Nat Rev Mater 6, 374–376 (2021), https://doi.org/10.1038/s41578-021-00305-z). Animal models do still represent the gold standard. However, they are limited by interspecies differences and the resulting poor prediction of human physiological and pathological conditions. To close this translational gap, human in-vitro disease models are increasingly being developed (Loewa, A., Feng, J.J. & Hedtrich, S. Human disease models in drug development. Nat Rev Bioeng 1, 545–559 (2023), https://doi.org/10.1038/s44222-023-00063-3).
These enable
- to gain more precise and comprehensive scientific insights into the molecular mechanisms that underlie diseases,
- to handle and repeat experiments easily with very good reproducibility and
- to ensure a high relevance of the results by reproducing essential aspects of tissue and organ complexity.
The use of suitable disease models in (pre-)clinical research increases the success rate of clinical implementation, reduces the costs of drug development and results in increasingly effective active ingredients (Loewa, A., Feng, J.J. & Hedtrich, S. Human disease models in drug development. Nat Rev Bioeng 1, 545–559 (2023), https://doi.org/10.1038/s44222-023-00063-3).
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB