PEG - biocompatible all-round talent
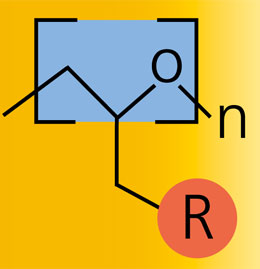
Polyethylene glycol (PEG), is non-toxic, non-immunogenic, hydrophilic and highly elastic. Due to these properties, the polymer is used in a wide range of applications in medical technology products, pharmaceuticals, the chemical and cosmetics industry and tissue engineering. The application usually requires a binding or cross-linking of the PEG. The network density has to be adjusted over the chain length of the PEG, because the functional groups are located at the chain ends. Commercially end group functionalized PEGs are available for this purpose, but the choice of available chain lengths is very limited.
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB