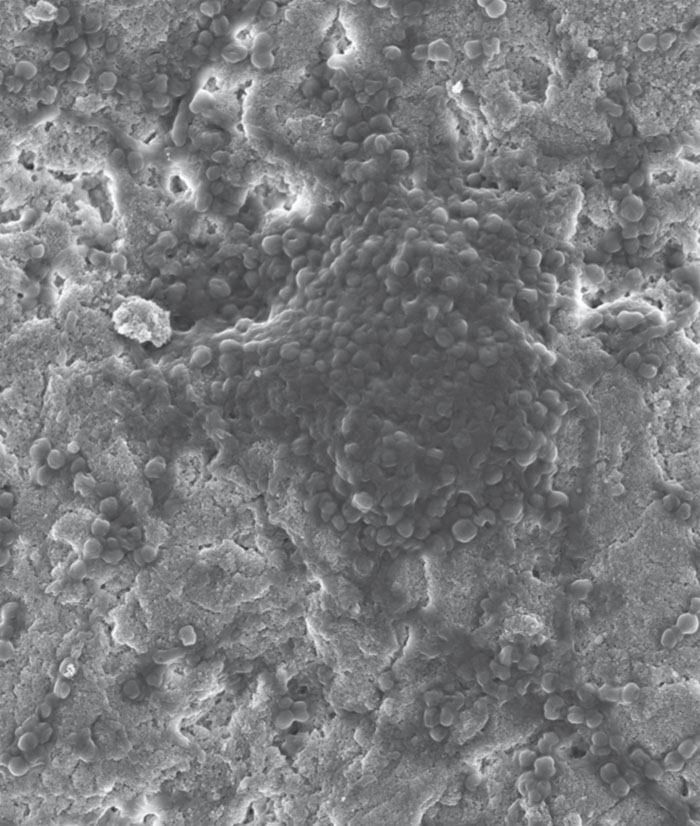
Several new formulations of chemically-modified (with or without surface modifications) nanocrystalline apatites were synthesized and fully characterized. The conditions enabling us to obtain single-phased apatite systems were retained and tests for antibacterial effects and cytotoxicity were carried out. Non-doped systems served as a reference. The apatite nanocrystals obtained showed high surface reactivity, especially through a hydrated surface layer. The influence of synthesis parameters, particularly the amount of antibacterial agent used per formulation and subsequent treatments was thoroughly investigated, especially in view of future possible areas of application.
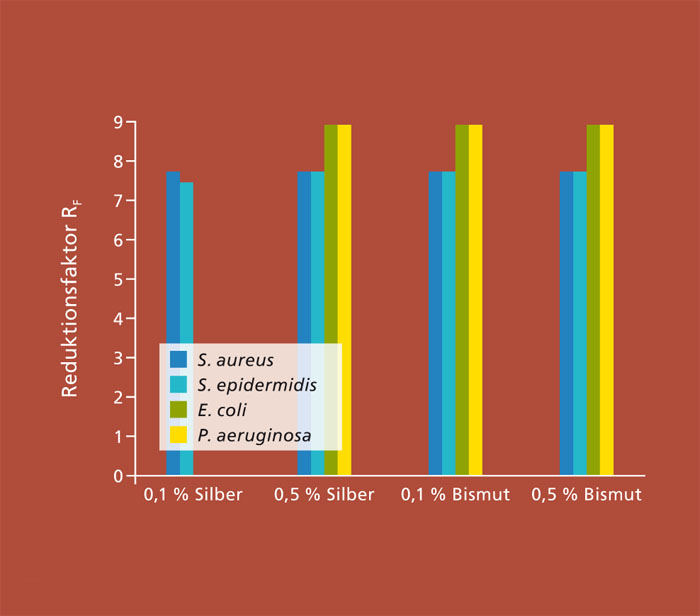
All the systems developed in the project were screened for antibacterial properties and cytotoxicity and the results were compared to determine the most promising formulations for future development to an industrial level. The left figure shows several selected screening results. The figure shows the reduction factor RF, which is calculated from the starting cell count used and the re-cultivable cells (RF=log (starting cell count) – log (number of re-cultivable cells)). A high starting cell count of between 107 cells/ml for Staphylococci and 109 cells/ml for E. coli and P. aeruginosa was reached on silver and bismuth-doped CaP apatite in high-concentration screening. The reduction factor RF shown in the diagram specifies the reduction in viable and reproductive cells in logarithm form. The maximum possible value corresponds to the number of starting cells used and therefore the total inactivation of the cells was accomplished. It was possible to fully inactivate the Staphylococci with the levels of doping shown. E. coli and P. aeruginosa show no impairment at 0.1 percent silver (RF = 0), while at only 0.5 percent silver both bismuth concentrations were equally fully inactivated.
Preliminary in vivo implantation tests were launched at the end of the project to investigate the potential impact of the best formulations on osteogenesis.

 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB