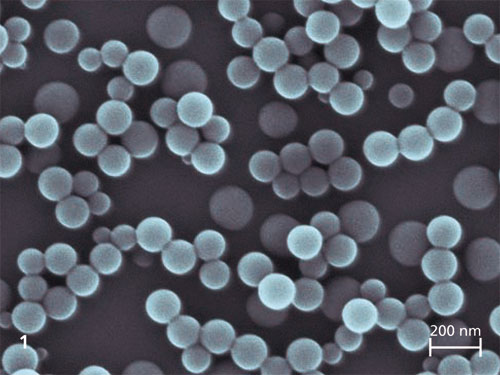
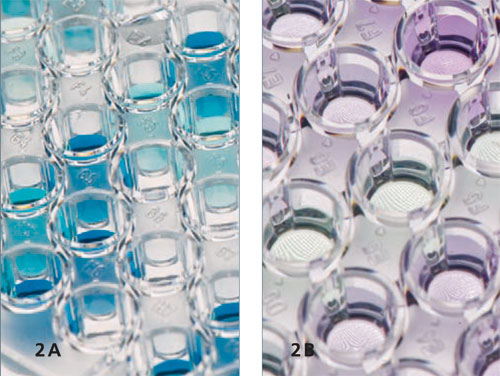
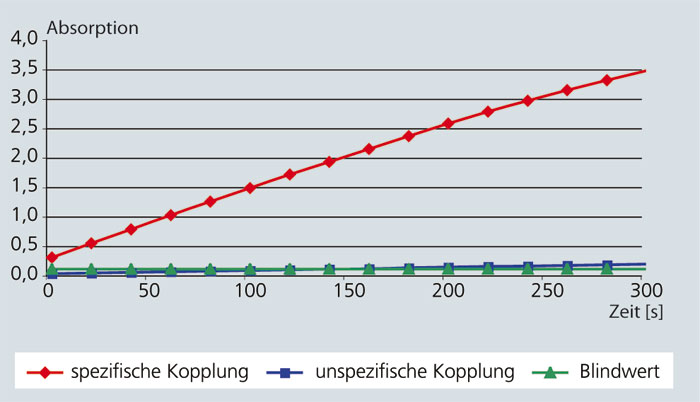
Enzymes are versatile biocatalysts that are increasingly used in industrial applications. However, the technical application of an enzyme is often limited by poor long-term stability under real process conditions and by difficulties in recycling. These weaknesses can be circumvented by immobilising the enzymes. In addition, immobilisation offers the possibility of both improving the catalytic properties and avoiding protein contamination in the product.
Benefits for the consumer
The motivation for using intelligent packaging materials is to increase safety for the consumer. With the help of packaging, consumers will in future be able to check the shelf life and quality of the food on offer directly while shopping.
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB