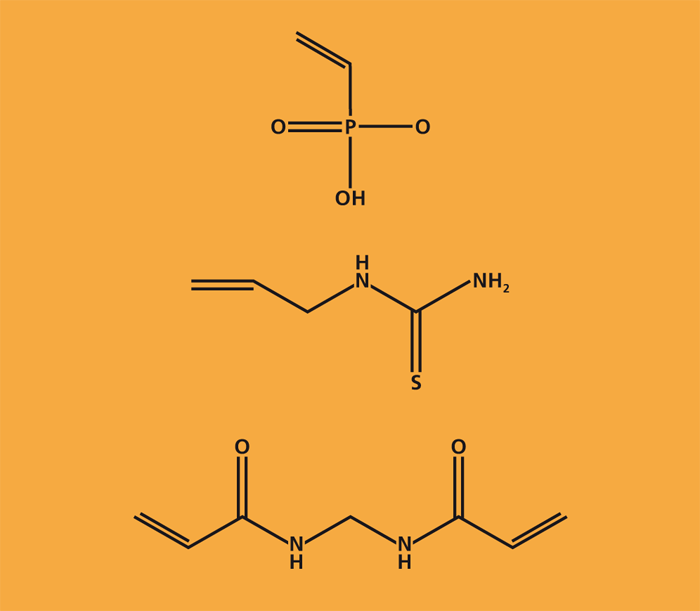
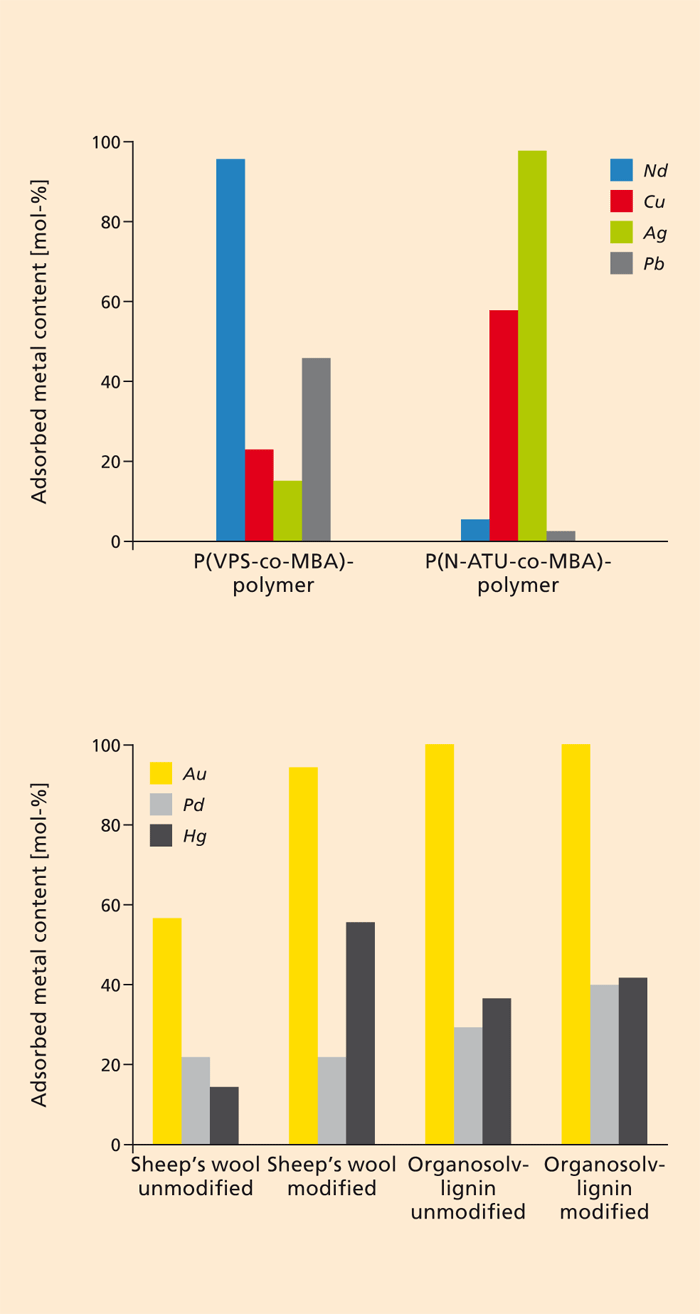
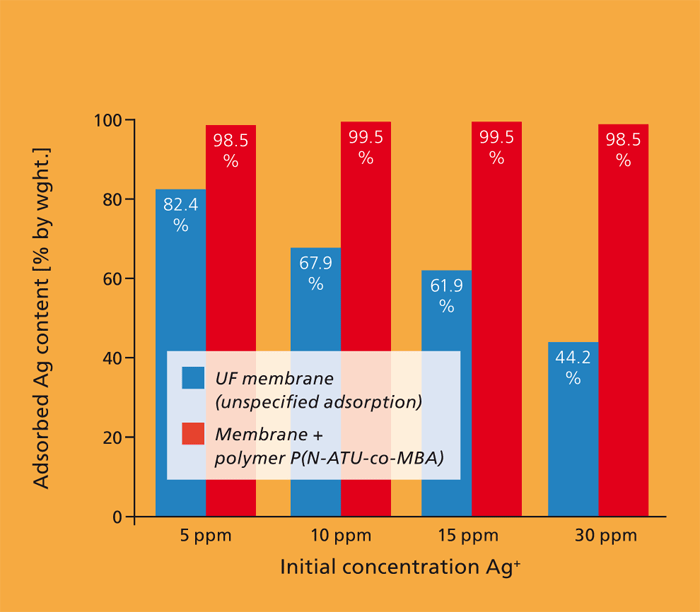
Waste as a source of raw materials
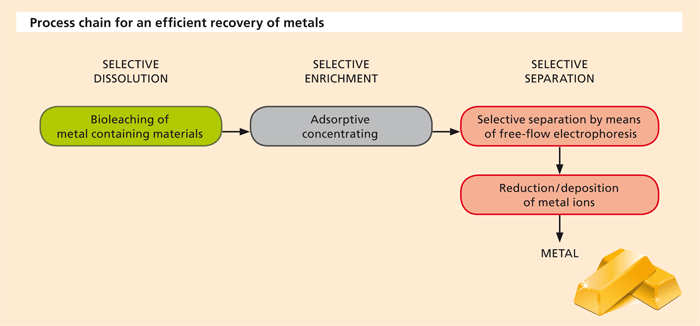
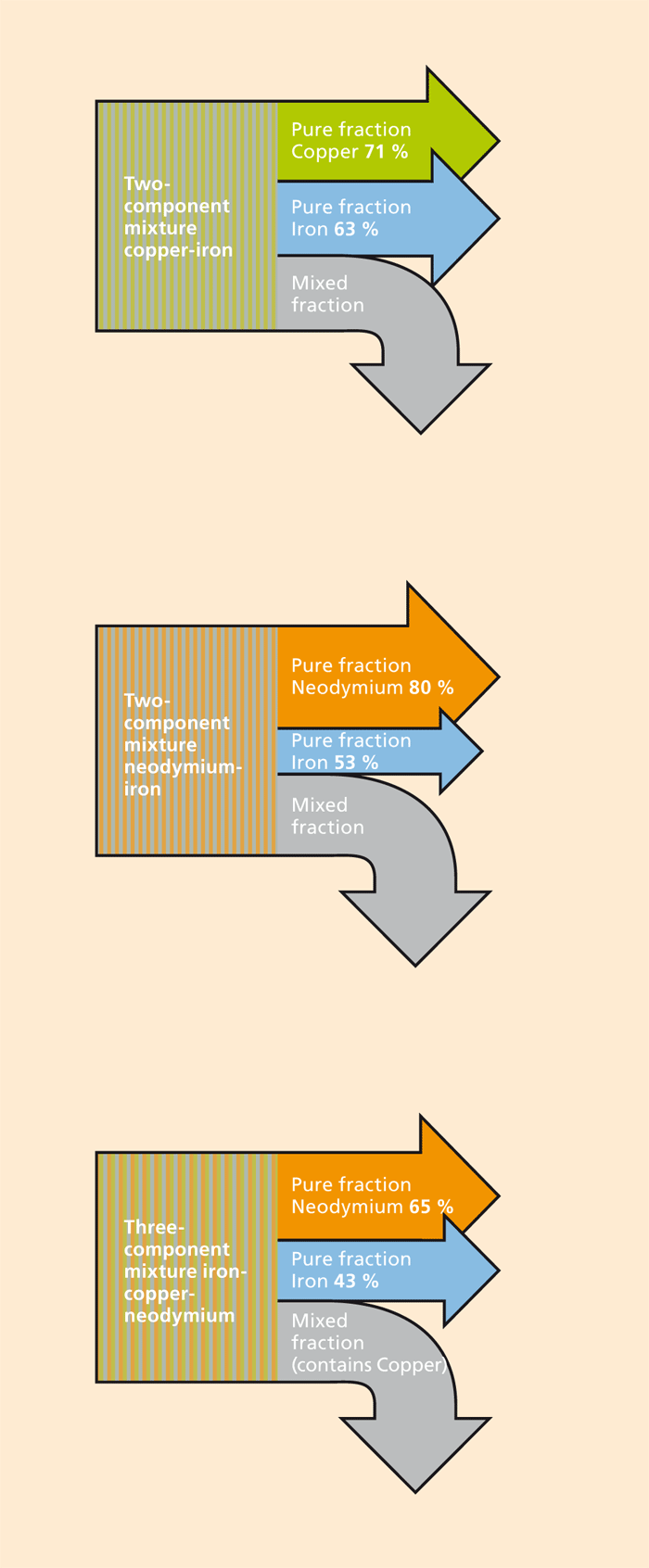
The recycling of raw materials, in particular special metals, because of their value (precious metals), their availability (rare earths) or their toxicity (heavy metals) is of great importance, both for industrial production and for the environment. Process and wastewater streams, for example from alkaline solution baths in the electroplating industry, and also landfill leachate, may contain significant quantities of dissolved metals. Also in the recycling of solids such as waste electronic equipment or ashes from combustion processes, the dissolution of metals (leaching) in bioreactors (bioleaching) can be an efficient method of transferring these metals to an aqueous solution. After that, further processes such as “enrichment”, “separation” and “deposition” are necessary to recover a utilizable metal. The design of these process steps is of decisive importance for the efficiency and sustainability of the whole process.
Need for new technologies
Economical and ecological efficiency on the industrial scale is only possible to a limited extent with the technologies available today, especially when there are only low concentrations of the metal ions in a solution. Of course, there are technologies for the selective separation of individual metals from a solution. However, these technologies are generally very cost-intensive, and neither environmentally friendly nor universally applicable. Furthermore, the precision of separation is not sufficient to achieve a quality equal to that of the primary material. To close the material cycles within production processes and in recycling there is therefore a great need to develop new technologies that are efficient, easy to integrate and can be applied flexibly to various groups of metals.
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB