PEG – a biocompatible all-round talent
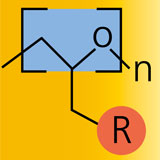
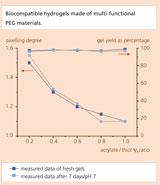
Polyethylene glycol, PEG, is non-toxic, not immunogenic, hydrophilic and highly elastic. Due to these qualities the polymer is a much sought after ingredient in medical products, pharmaceuticals, the chemical and cosmetic industries as well as in tissue engineering. Its application normally requires that PEG be linked to a matrix or cross-linked. This process involves adjusting the cross-link density by means of the chain length of PEG since the functional groups are located at the end of the chain. Such end-group functionalized PEG is commercially available; however, the choice of chain lengths is extremely limited.
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB