Required: Efficient separation of small molecules
Nowadays various types of membranes for water filtration are already available through commercial channels. A common feature of these membranes is that substantially different separation cut-offs are used for size exclusion. On the other hand, the underlying porous structure, which provides a highly specific surface, remains unused. Membranes for nanofiltration (NF) and reverse osmosis (RO) can in fact partially retain molecular and ionic substances. However, high pressures are necessary for this, which pushes up both the investment and the operating costs.
In principle adsorbers can be used to remove molecular contaminants. Typical adsorber materials are microporous, so as to provide a large specific surface for adsorption. A disadvantage of these materials is the limited mass transport, since the micropollutants have to diffuse into the inner porous structure of the adsorbents.
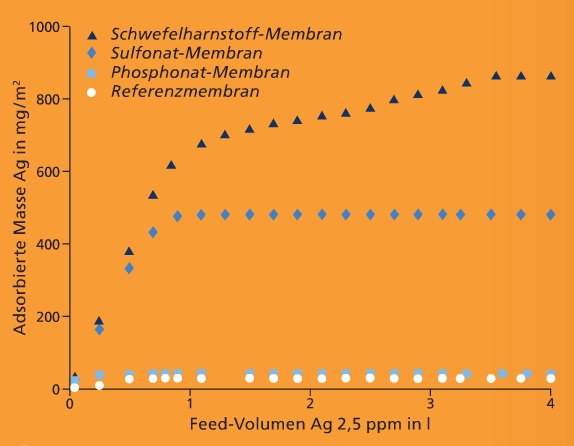
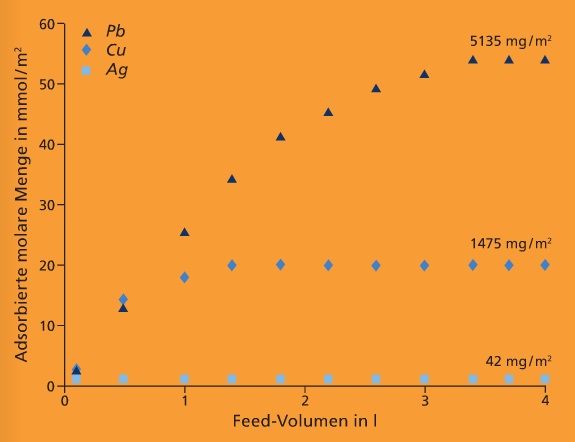
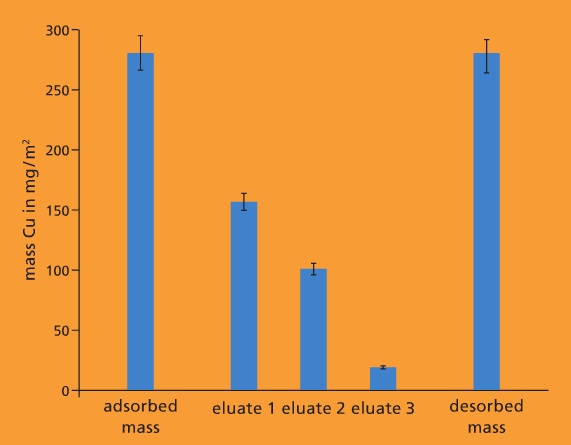
There is therefore a need for new integrated separation systems. For this purpose we are developing mixed-matrix membranes that, in addition to their filtration function, can adsorptively bind substances dissolved in water.
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB