The need for phosphorous fertilizers is growing with the increasing global demand for foodstuffs. Fraunhofer IGB has developed and patented a novel process for recovering ammonium and phosphate from wastewater. With our ePhos® process, the substances are precipitated in an electrochemical process as plant-available magnesium ammonium phosphate (struvite). Struvite is a high-quality slow-release fertilizer and can be used directly as a fertilizer in agriculture.
ePhos® – Electrochemical phosphorus recovery
Our approach

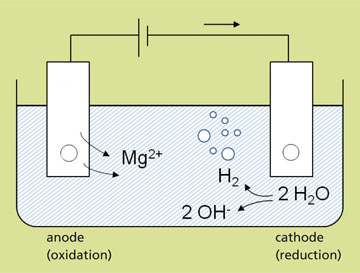
In order to recover phosphorus (P) and nitrogen (N) from a liquid phase (process or waste water), crystallization as struvite (magnesium ammonium phosphate hexahydrate: (NH4)Mg[PO4]*6 H2O) is an option. In the conventional precipitation of struvite, magnesium must be added as a limiting reactant in the form of a solution of MgCl2, Mg(OH)2 or MgO. In addition, the pH value must be raised with sodium hydroxide solution, for example.
In the ePhos® process developed at Fraunhofer IGB, phosphate precipitation is carried out electrochemically.
Advantages
The main advantage here is that the chemicals mentioned do not need to be added at all. The magnesium required for struvite formation is added by means of a sacrificial anode in an electrolysis cell.
The energy requirement for the process is comparatively low. Indeed, the higher the concentration of ions in the wastewater, the lower the energy requirement. In pilot tests, the energy required was in the region of 0.78 kilowatt hours per cubic meter of wastewater.
Struvite – a high-quality fertilizer
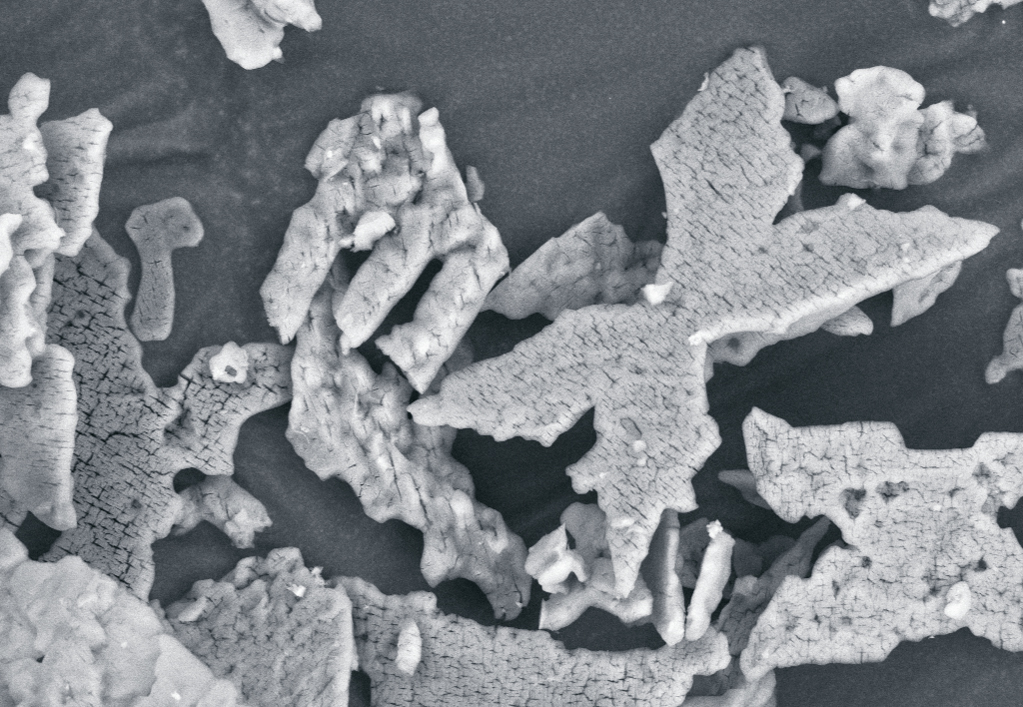
The recovered product struvite can be used directly in agriculture as a high-quality, slow releasing fertilizer. Struvite was tested in plant-pot experiments showing excellent results. Plant yield and plant nutrient-uptake with struvite were up to 4 times higher than with commercially available mineral fertilizer (calcium ammonium nitrate and triple superphosphate). This result demonstrates that struvite is easily plant-available and has a positive effect in plant growth.
Our development
Process principle
Electrochemical phosphorus precipitation takes place in an electrolytic cell consisting of an inert cathode and a sacrificial anode made of magnesium. As a result of the cathodic reduction, water molecules are split, forming OH– ions and hydrogen (H2) which is released. The OH– ions increase the pH value of the wastewater, which remains constant at pH 9. The ePhos® process therefore eliminates the need to adjust the pH value by dosing alkaline solutions for the precipitation process. Oxidation takes place at the anode: Magnesium ions dissolve and react with the phosphorus and nitrogen contained in the water to form struvite.
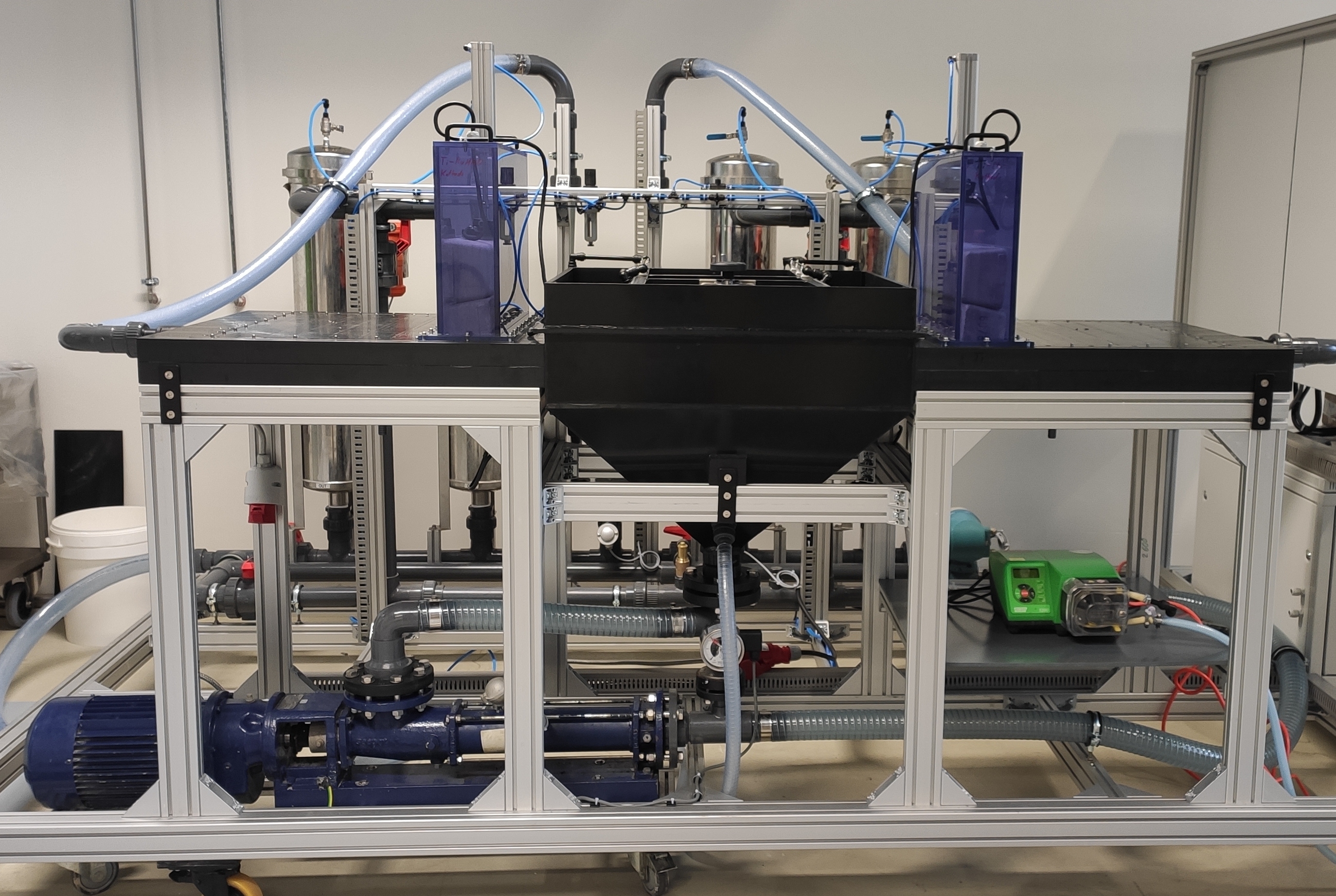

Feasibility study in a pilot plant
In the course of a feasibility study the process was tested using a pilot plant with a flow rate of up to 1 m3/h at a sewage treatment plant with biological phosphorus elimination. The phosphorus concentration in the centrate water was reduced by an average of 180 mg/L to 20.8 mg/L. Thus, the average phosphorus elimination rate from the centrate water of the digested sludge dewatering and the phosphorus conversion to struvite respectively was more than 80 percent.
The phosphorus load that no longer has to be treated when the filtrate water is recirculated, decreases by 37 percent; this amounts to 9284 kilograms annually and results in a reduction of sludge production by 7 percent. The design of the process for the client’s plant shows that the electrochemical phosphate precipitation would require approx. 10 tons of magnesium in the form of sacrificial electrodes per year. From this, approx. 73 tons of struvite per year would be obtained which can then be used directly as a fertilizer. The total quantity of chemicals that would have to be used at the treatment plant would decrease by 40 tons or 20 percent per year.
Demonstration operation at the Erbach wastewater treatment plant
The ePhos® process is currently being piloted at the Erbach wastewater treatment plant as part of the “RoKKa – sewage sludge as a source of raw materials and climate protection at wastewater treatment plants” project funded by the Baden-Württemberg Ministry of the Environment, Climate Protection and the Energy Sector and the EU.
Applications
In addition to wastewater treatment, the process is also suitable for the food industry and agricultural biogas production, whose wastewater is rich in ammonium and phosphate.
Further process modules will be added to the ePhos® process in order to recover ammonium in the future. For this purpose, we are working on our AmmoRe process to investigate the process of chemical transmembrane absorption to not only remove ammonia from wastewater, but also to recover it as a fertilizer (ammonium sulfate solution).
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB