Our expertise in the field of medical technology for the production of functional surfaces, material development and surface analysis round off the IGB's range of services. The focus here is on coating technologies and material developments through to biological inks for medical technology companies. In addition, we establish plasma and UV sterilisation processes for sterilisation and removal of pyrogenic residues with regard to maximum effectiveness and material protection.
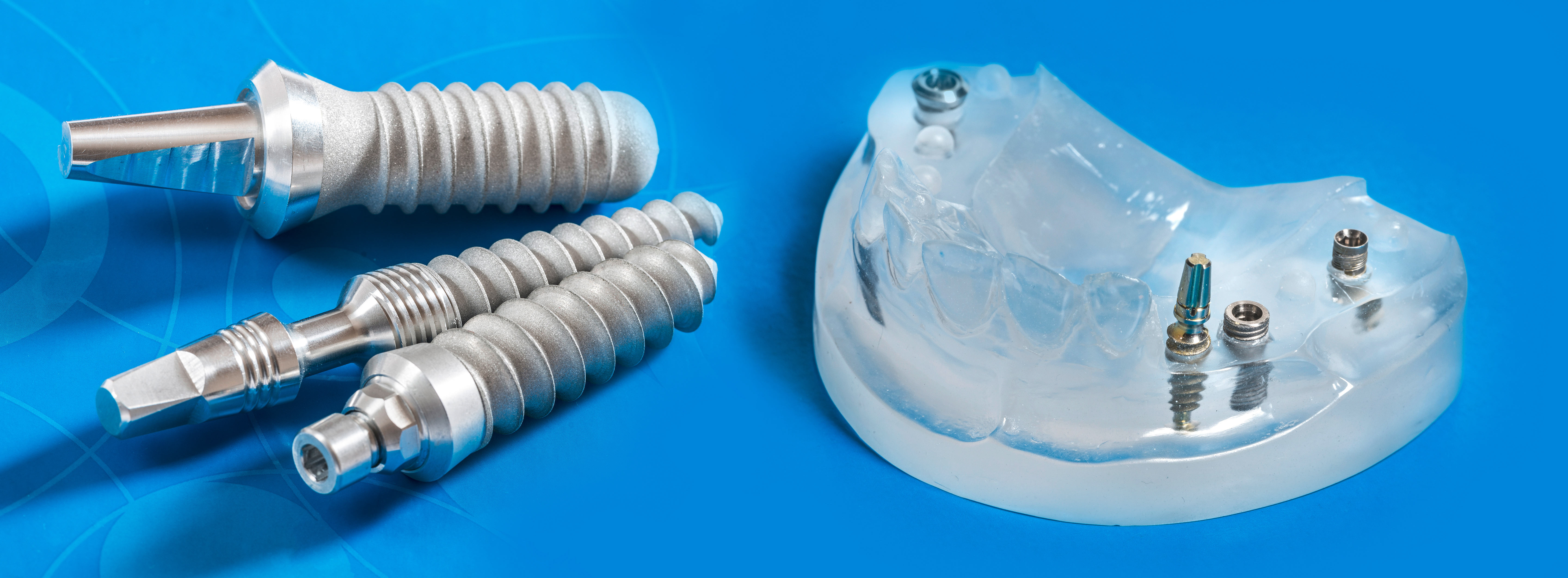
Medical engineering
Coatings for optimized material properties
Control of biological processes at the interface from implant to biological system
We modify implant materials using plasma technology, for example, to avoid the unspecific adsorption of proteins. Specific biological coatings, including those with proteins, enable human cells to grow faster and thus promote faster healing of implants. The biocompatibility of the surfaces also ensures the functionality of the adjacent biological tissue in the case of artificial extracellular carrier structures.
Biomaterials and bio-inks
In the biomaterials research area, we investigate specific cell-material interactions especially for implants and develop and optimize materials such as hydrogels. One focus is on the investigation and reproduction of the extracellular matrix – quasi as the optimal biomaterial based on nature.
Besides biological carriers, Fraunhofer IGB also develops miniaturized tubes – as supply systems for larger tissue models. The adaptation of additive processes to the processing of biological materials and cells for personalized implants on the one hand and the development of so-called bio-inks – formulations of biomolecules that can first be dispensed and then cross-linked to form stable, tissue-like matrices – are the subject of further current research work.

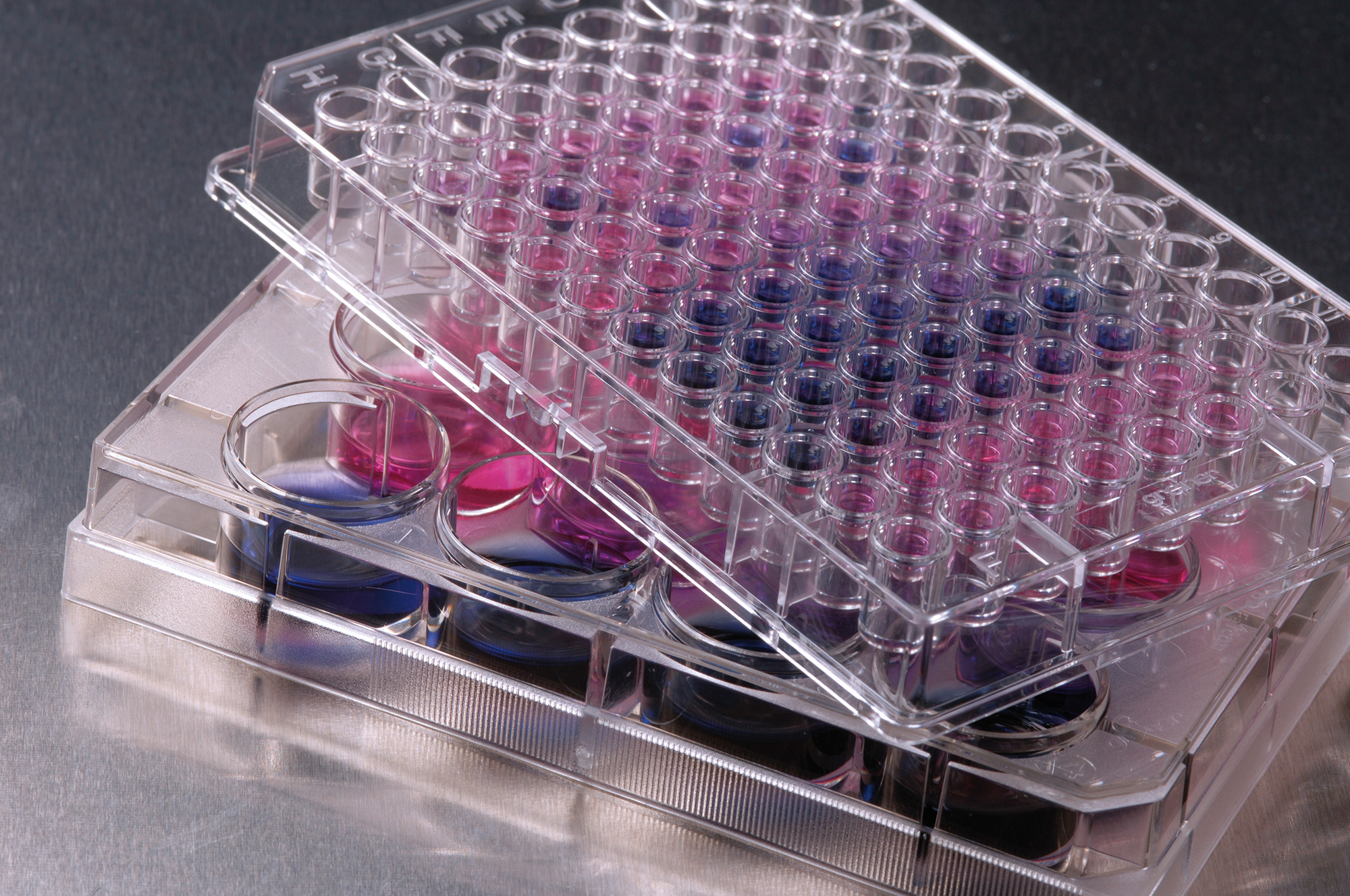
Detection of pyrogens
We have developed cell-based assay systems to detect pyrogens – e.g. bacterial cell components that cause febrile reactions up to shock states in humans. These are suitable for testing medical devices for the absence of pyrogens and can supplement or replace existing tests.
Plasma and UV sterilization processes
For the disinfection and removal of pyrogenic residues, we establish plasma and UV sterilization processes, for example for reusable sterile containers, with a view to maximum effectiveness and material protection.

Determination of free radicals in irradiated medical devices
The World Health Organization (WHO) recommends gamma sterilization for the sterilization of heat-sensitive drugs and medical devices. In this process, the products are irradiated with high-energy gamma rays, which destroy the DNA of microorganisms and kill the germs. However, the irradiation also produces undesirable free radicals. We quantify the amount of free radicals quickly and reliably using electron spin resonance spectroscopy.
We also use electron spin resonance spectroscopy to determine reactive oxygen species (ROS) and nitrogen monoxide (NO) in biological systems, e.g. cells or blood.
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB