Veterinary medicines are often used without prior testing for efficacy, or human therapeutics are used even though they are not actually suitable for such purposes. This also applies to dermatological problems in dogs, which are particularly common. At Fraunhofer IGB, we have successfully developed an in-vitro canine skin equivalent for standardized testing of veterinary therapeutics and care products. For this purpose, cells (fibroblasts, keratinocytes) were isolated from canine skin biopsies, which are obtained during medically necessary operations. The cells were genetically immortalized and used to build canine skin equivalents that are physiologically very similar to in-vivo canine skin.
WowWowSkin – Establishment of in-vitro canine skin equivalents
In-vitro canine skin equivalents for testing veterinary therapeutics

Due to the large amounts of money spent on treatments in the companion animal sector, the global veterinary medicines market is a steadily growing market, with an estimated value of US$18.5 billion for 2021 [1]. However, compared to human medicine, the market for veterinary medicines is poorly regulated. Therapeutics are used in this context even without prior testing for efficacy, often with minimal or no evaluated benefit. In addition, human therapeutics are often applied to animals, although pharmacodynamics and toxicological effects differ, sometimes significantly, on a species-specific basis. Therefore, species-specific testing is increasingly required for efficacy and risk assessment of veterinary therapeutics. In order to avoid controversial animal experiments at the same time, in- vitro test methods are suitable for this purpose.
Standardized testing of therapeutics and care products for dogs
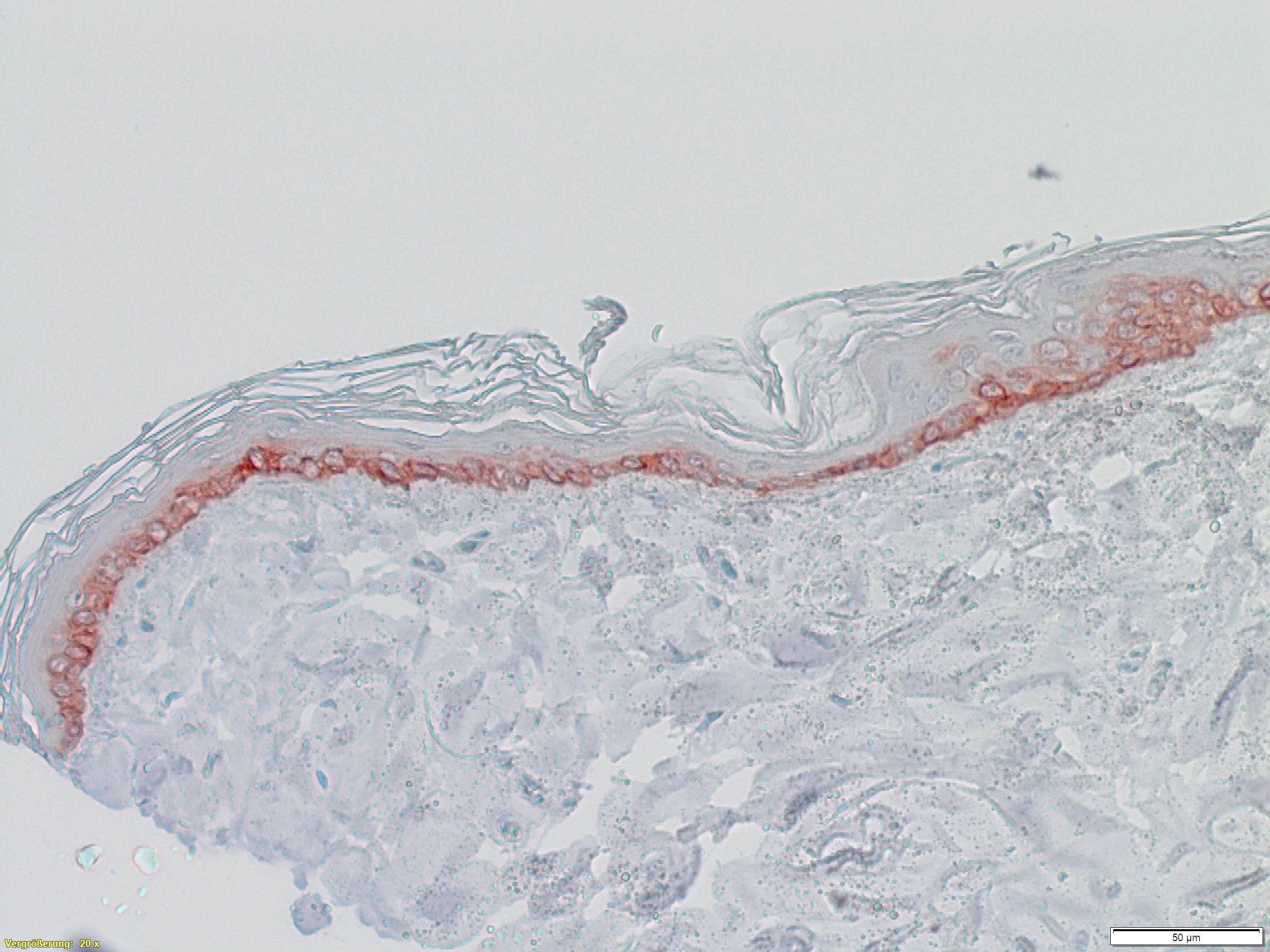
Dermatological problems of pets, especially dogs, are among the most frequently occurring diseases in veterinary clinics. In the WowWowSkin project, the IGB is therefore developing an in- vitro model for canine skin (see Fig. 1) in order to be able to test therapeutic agents and care products for dogs in a standardized manner. To this end, (1) primary cells are isolated from canine skin biopsies, (2) they are genetically immortalized, and finally (3) used to construct canine full-thickness skin equivalents (see Fig. 2). The isolation of primary cells and their immortalization as well as the establishment of in- vitro skin models has been successfully performed with human material for decades in the Innovation Field Cell and Tissue Technologies. Based on this expertise, essential steps were adapted and optimized for the isolation of primary cells from canine biopsies. Primary keratinocytes, fibroblasts, endothelial cells and melanocytes were successfully isolated and expanded. These were immortalized by stable transfection with suitable plasmids to finally establish in in-vitro epidermis, dermis, and full-thickness skin models.
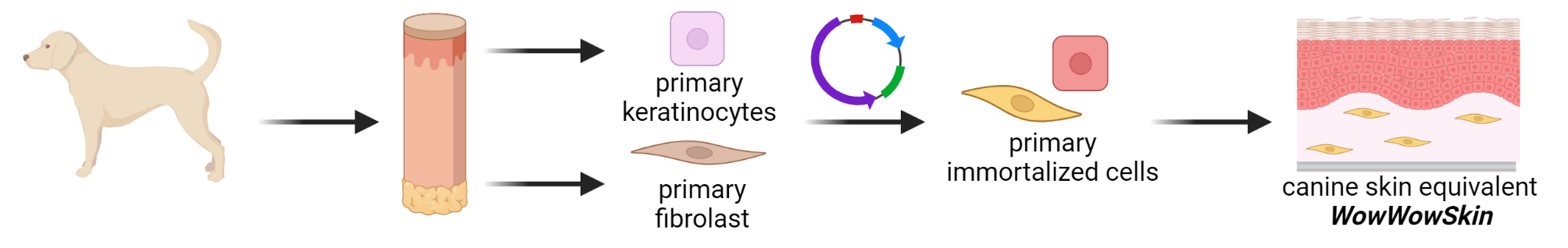
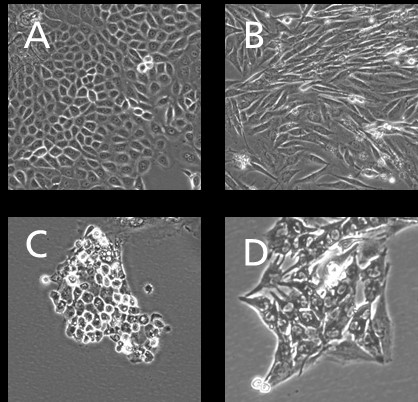
Reproducible complex canine skin model
The final canine full-thickness skin model, consisting of different immortalized cell types, is characterized on the one hand by a high donor-independent reproducibility and on the other hand by its cellular complexity. With the development of a canine in- vitro skin model, the IGB internally funded garage project WowWowSkin enables the IGB to enter a new strategic field of animal in- vitro skin models, addressing the testing of veterinary therapeutics, especially for the treatment of zoonotic skin diseases, of grooming products as well as of flea and tick prevention products.
The canine skin equivalents are available at Fraunhofer IGB for the testing of active ingredients and care products and have the following characteristics:
- Representation of typical physiological skin conditions in the canine skin model, e.g. epidermal differentiation and barrier function
- Use for medium to high throughput drug screening
- Accurate and reproducible detection of substances that cause typical pathological skin conditions (e.g. skin irritation)
Services offer and collaboration
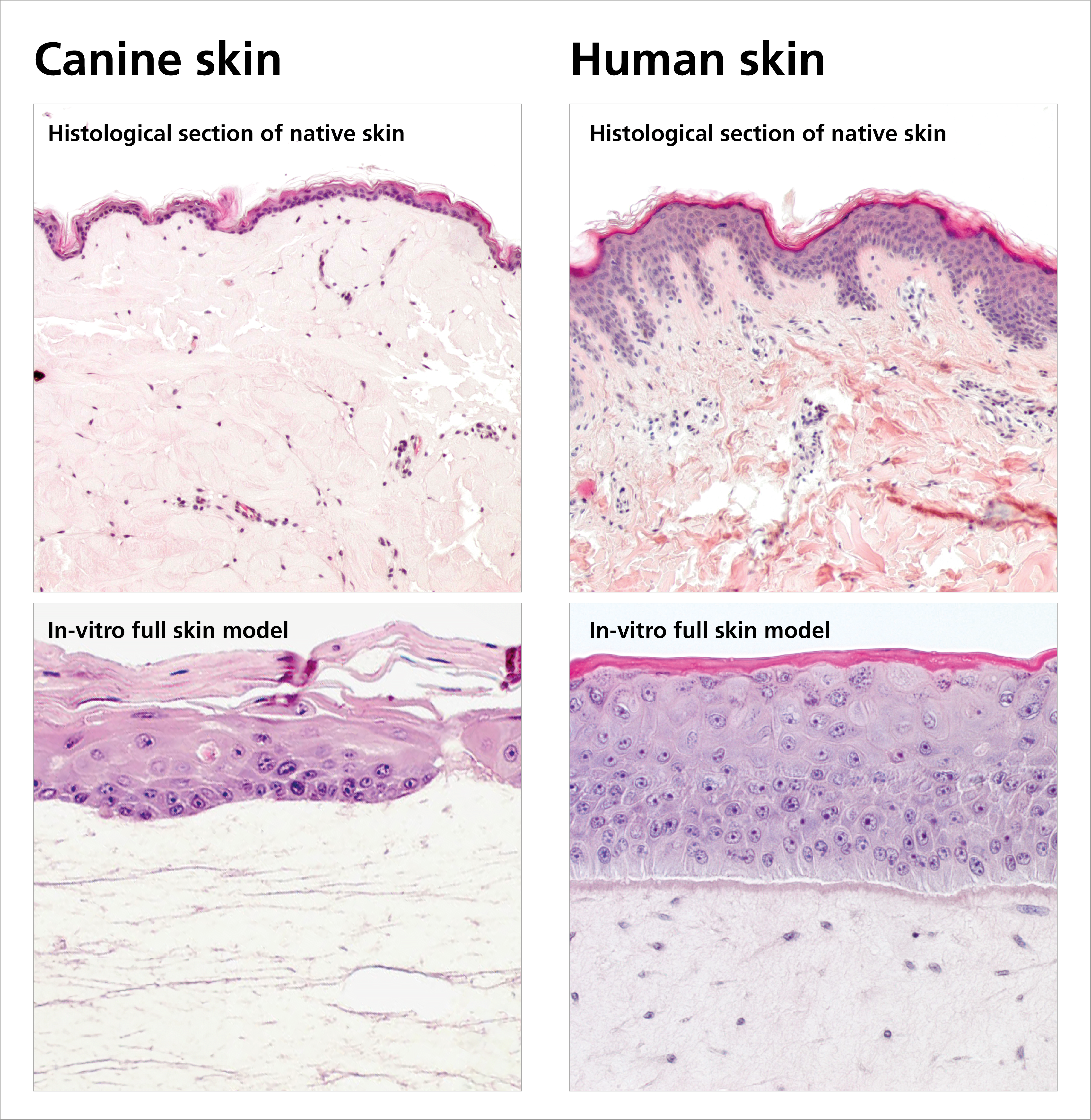
Advantages and technological readiness
Due to the use of immortalized cells, the in-vitro canine skin equivalent of Fraunhofer IGB (TRL 9) differs from the only competing product by a high donor-independent reproducibility with an in-vivo like differentiation of the cells. The testing of active ingredients can be carried out quickly, easily and reproducibly at any time and replaces in-vivo investigations. In addition, the canine skin model is suited for various types of active ingredient application (topical, systemic).
Collaboration
Due to the high level of technological readiness, we can offer various services and further developments. Feel free to contact us to discuss your needs and our services.
- Testing of veterinary pharmaceuticals and canine fur care products in our laboratories at Fraunhofer IGB
- Transdermal ingredient studies
- Skin penetration studies
- Mode of action of pharmaceuticals and active ingredients
- Wound healing studies
- Toxicological evaluation of chemicals and products
Literature
[1] MarketsandMarketsTM (2021) Companion animal pharmaceuticals market, Report Code MD 7870, https://www.marketsandmarkets.com/Market-Reports/companion-animal-pharmaceutical-market-252218886.html und https://www.bft-online.de/der-verband/tierarzneimittelmarkt (retrieved 17. March 2022)
Project information
Project title
WowWowSkin – Establishment of in-vitro canine skin equivalents
Project duration
June 2022 – June 2023
Project coordination
Funding
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB