Surfactants are produced worldwide in the order of 18 million tons and used as detergents, emulsifiers, dispersants and foaming agents in a wide variety of sectors, from the textile industry to mining. Surfactants are synthesized both on the basis of fossil raw materials and from renewable raw materials, such as palm oil, using chemical processes. However, the structural diversity of such chemically produced surfactants is limited and the sustainability of the tropical vegetable oils used is currently the subject of controversial debate.
Biosurfactants – Production and optimization
Biosurfactants from renewable raw materials

Privacy warning
With the click on the play button an external video from www.youtube.com is loaded and started. Your data is possible transferred and stored to third party. Do not start the video if you disagree. Find more about the youtube privacy statement under the following link: https://policies.google.com/privacy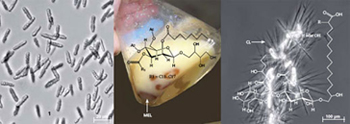
Biosurfactants – Bacterially produced surfactants
Several microorganisms naturally form a multitude of surface-active substances, so-called biosurfactants, which cover a broad spectrum of chemical structures. These include glycolipids, lipopeptides, lipoproteins and heteropolysaccharides. The properties of these biosurfactants are comparable or even superior to many synthetic surfactants in terms of surface activity and degradability and are therefore of interest for many industrial applications.
Improved manufacturing and processing processes, more efficient production strains and the increased demand for "green" products have brought some biosurfactants to market maturity in recent years. One example of this is the sophorose lipid from Starmerella bombicola, which is now produced by various surfactant manufacturers as an additive for household cleaners and dishwashing detergents.
Glycolipids with great potential
Two classes of biosurfactants that have also proven to be promising detergents, emulsifiers and active ingredients in cosmetics, crop protection and industrial applications are cellobiose lipids (CL) and mannosylerythritollipids (MEL). They are formed by smut fungi of the genera Moesziomyces and Ustilago in larger quantities. Their antimicrobial properties also make them interesting for use in clinical and pharmaceutical applications.
Recently, another class of microbial biosurfactants, so called polyol lipids (PL), have been investigated in our research group. These biosurfactants can also be produced from sugars via fermentation with Rhodotorula species.
However, industrial production of these biosurfactants still requires improvements in the yield during fermentation and in the reproducibility of the product composition. The aim of these investigations is to increase the productivity of cultivation and the use of renewable raw materials as substrates.
Range of services
- Fermentation and sample production of fermentatively produced biosurfactants
- Chemical-enzymatic modification
- Scale-up of fermentation up to max. 10 m3 at the Fraunhofer Center for Chemical-Biotechnological Processes CBP, Leuna
Goals and strategies
Fraunhofer IGB deals with the optimization of the biotechnological synthesis of glycolipids, in particular cellobiose lipids (CL) and mannosylerythritollipids (MEL) using different fungi and different substrates such as mono- and disaccharides, vegetable oils or residues. In this way, tailor-made structural mixtures are to be created and tested for their application-specific suitability.
The objectives are the characterization and optimization of biosurfactants for use in cleaning agents, in cosmetics or for special applications in industry, as well as efficient fermentative production of the biosurfactants. We are pursuing various approaches for the optimization of biosurfactants and the fermentation process such as:
- Optimization of bioprocess control strategies
- Enzymatic modification of the produced biosurfactants
- Genetic modification of the specific metabolic pathways of the microorganisms used
Results and prospects

Through process optimization of the manufacturing process for the two biosurfactants CL and MEL, we currently achieve product concentrations of > 20 g/L for CL and 50 g/L for MEL. These were transferred from the shaking flask to the reactor scale (1 L, 10 L, 42 L). Various cultivation methods, substrates and processing strategies were investigated.
The quantities currently produced are sufficient for comprehensive technical application investigations of the respective biosurfactants. By enzymatic and chemical modification, the hydrophilic and hydrophobic properties of the glycolipids produced were specifically altered, thus increasing the emulsion-stabilizing effect and solubility. The fermentation process is currently being further improved in order to achieve a production with the highest possible space-time yield.
Strategies for avoiding foam
We are currently investigating various methods to avoid foam formation during fermentative MEL production. For CL, a fraction with a high cellobiose lipid concentration was collected by continuously separating the foam produced during fermentation. If this foam fraction is purified directly, only seven percent of the solvent quantity is required for the extraction of the cellobiose lipids, compared to conventional purification of the entire reactor content.
Software-assisted process optimization
The individual process steps are evaluated by means of a life cycle analysis and a techno-economic assessment during the course of development. With the aid of these assessments, process bottlenecks are identified and validated experimentally.. The findings obtained serve to continuously improve the economy and ecology of the overall process.
Prospects
In parallel, we are researching genomes and transcriptomes of promising production strains using next-generation sequencing methods to elucidate the biosurfactant metabolism and its regulatory mechanisms and to change the product spectrum and productivity of the strains using metabolic engineering.
Publications
Oraby, A., Hug, D., Weickardt, I., Maerz, L., Nebel, S., Kurmann, J., Rupp, S., Tovar, G. E. M. (2023). Fermentation and recovery of cellobiose lipids using foam fractionation. Discover Chemical Engineering 3. https://doi.org/10.1007/s43938-022-00015-0
Oraby, A., Weickardt, I., Zibek, S., (2022). Foam fractionation methods in aerobic fermentation processes. Biotechnology and Bioengineering, 119. https:// doi.org/10.1002/bit.28102
Beck, A., Vogt, F., Hagele, L., Rupp, S., and Zibek, S. (2022). Optimization and Kinetic Modeling of a Fed-Batch Fermentation for Mannosylerythritol Lipids (MEL) Production With Moesziomyces aphidis. Front Bioeng Biotechnol 10, 913362. https://doi.org/10.3389/fbioe.2022.913362
Oraby, A., Rupp, S., and Zibek, S. (2022). Techno-Economic Analysis as a Driver for Optimisation of Cellobiose Lipid Fermentation and Purification. Front Bioeng Biotechnol 10, 913351
Oraby, A., Werner, N., Sungur, Z., Zibek, S. (2020). Factors Affecting the Synthesis of Cellobiose Lipids by Sporisorium scitamineum. Front Bioeng Biotechnol 8. https://doi.org/10.3389/fbioe.2020.555647
Beck, A., and Zibek, S. (2020). Mannosylerythritollipide — mikrobielle Biotenside aus dem Bioreaktor. BIOspektrum 26, 100-102. https://doi.org/10.1007/s12268-020-1332-3
Beck, A., Haitz, F., Grunwald, S., Preuss, L., Rupp, S., and Zibek, S. (2019). Influence of microorganism and plant oils on the structure of mannosylerythritol lipid (MEL) biosurfactants revealed by a novel thin layer chromatography mass spectrometry method. J Ind Microbiol Biotechnol 46, 1191-1204. https://doi.org/10.1007/s10295-019-02194-2
Beck, A., Werner, N., and Zibek, S. (2019). "Chapter 4 - Mannosylerythritol Lipids: Biosynthesis, Genetics, and Production Strategies," in Biobased Surfactants (Second Edition), eds. D.G. Hayes, D.K.Y. Solaiman & R.D. Ashby. AOCS Press), 121-167. https://doi.org/10.1016/B978-0-12-812705-6.00004-6
Günther, M., Grumaz, C., Lorenz, S., Stevens, P., Lindemann, E., Hirth, T., Sohn, K., Zibek, S., and Rupp, S. (2015). The transcriptomic profile of Pseudozyma aphidis during production of mannosylerythritol lipids. Appl Microbiol Biotechnol 99, 1375-1388. https://doi.org/10.1007/s00253-014-6359-2
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB