Important synthesis building block 2,5-furandicarboxylic acid (FDCA)
2,5-furandicarboxylic acid (FDCA) and other dicarboxylic acids are important synthesis building blocks in the chemical industry due to their bifunctionality. 2,5-furandicarboxylic acid is also considered as a promising platform chemical with large market potential: the U.S. Department of Energy, for example, lists FDCA as one of the top 12 chemicals that can be produced from biomass. In a follow-up study, FDCA was also included in the top 10 due to its potential applications.
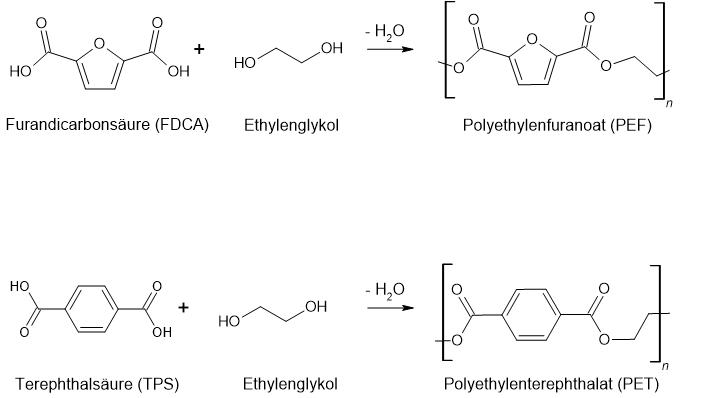
FDCA is considered so promising because of its high structural homology to terephthalic acid. Which is used to synthesize the widely used plastic polyethylene terephthalate (PET), whose annual market volume is approximately 40 million tons (2004 status).
Bio-based polyester PEF
Analogous to the polymerization of terephthalic acid to PET, FDCA can be polymerized to polyester polyethylene furanoate (PEF). FDCA, which can be produced from renewable resources, thus offers a sustainable substitute for petroleum-derived terephthalic acid in the production of bio-based polymers.
PEF is not only a bio-based plastic, but - unlike PET - it is also biodegradable. Haptically and visually, the polymer certainly resembles its petrochemical equivalent PET. PEF is not only characterized by its sustainability, but also exhibits significantly improved gas and water retention properties than PET: its water retention capacity is twice as high and it has six times and ten times better barrier properties to carbon dioxide and oxygen, respectively.
Biotechnological production of FDCA
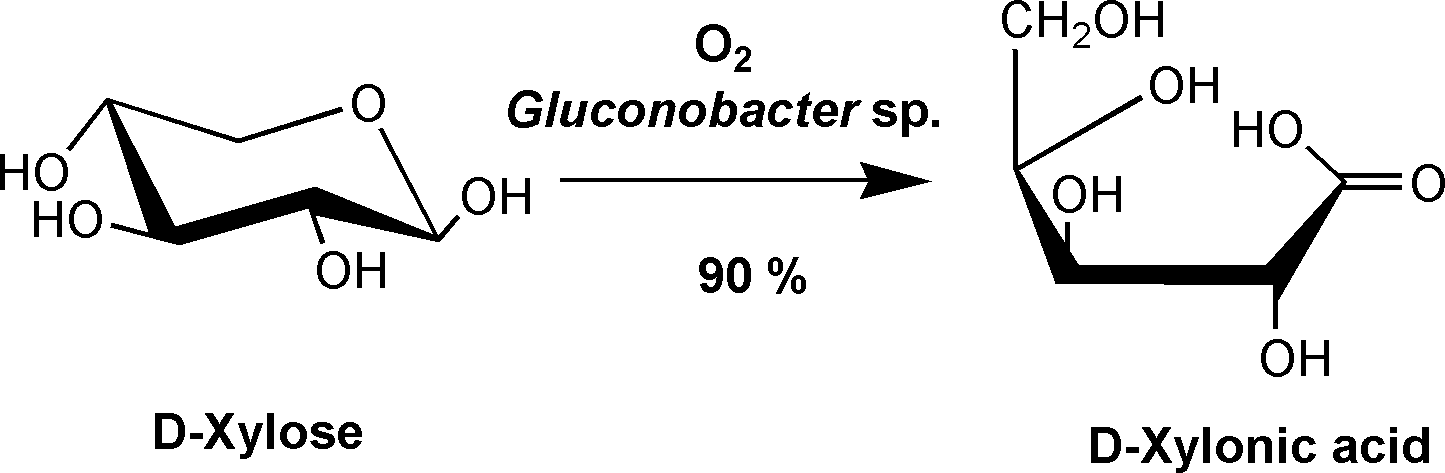
FDCA can be produced from a variety of feedstocks, but hydroxymethylfurfural (HMF) is the most promising because it allows for different synthetic routes such as a chemical or biological synthesis (microbial or enzymatic). Biocatalysis represents a promising approach. Advantages include mild reaction conditions, lower cost, higher selectivity, and environmental friendliness. However, biological production of FDCA is not yet established.
Therefore, the research activities of Fraunhofer IGB focus on the establishment of whole cell catalysis of HMF to FDCA. In the BioConSept research project, we were able to successfully establish whole-cell microbial catalysis using targeted feeding strategies of hydroxymethylfurfural obtained from lignocellulosic biomass, achieving a yield of more than 80 percent at a concentration of up to 20 g/L FDCA in the laboratory. The subsequent scale-up was carried out using model experiments with scalable reactors or fermenters in the laboratory. Using dimensionless metrics selected in this process, we were able to design and successfully run the processes in the larger scale pilot plant.
The biotransformation of HMF to FDCA is also the subject of the current KEFIP research project. The aim of the project is to develop a sustainable multi-stage process for the conversion of inulin-containing chicory root beets, an agricultural waste product. HMF is extracted from inulin of the root beet by hydrothermal dehydration and subsequently oxidized to FDCA. The IGB is working in the project on the extraction of inulin from the root beet and also on the microbial oxidation of HMF to FDCA. In the field of FDCA production, the Fraunhofer IGB was able to show that a biotransformation of HMF to FDCA is possible when using an HMF solution from chicory which still contains various impurities. By optimizing the fermentation protocol it was possible that the yield of FDCA was exactly the same and there was no difference compared to the use of a pure HMF solution, which is free of impurities.
Furthermore, in the KEFIP project, the IGB developed a novel feeding strategy for the HMF solution based on online data from the fermentation.
 Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB
Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB